WK95
- 139
- 1
How do I relate Fourier's Law of Heat Conduction for 1-D Heat Conduction with the Heat Conduction Equation in a large plane wall and energy balance equation?
Fourier
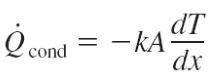
Energy Balance
energy in - energy out = system energy change
rate of energy in - rate of energy out = rate of system energy change
Heat Conduction Equation
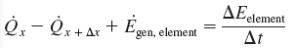
So is Q_dot.cond is the rate of heat transfer through a distance x by conduction due to a temperature difference. That means there is heat energy flowing from into the body from the higher temperature side and exiting through the lower temperature side. Does that mean Q_dot.cond = Q_dot.x + Q_dot.x+deltax (The first two terms of the Heat Conduction equation except they are summed) which is the sum of energy in one direction from high to low.
Asked another way, if I were considering heat conduction through some body, how is the energy balance equation applied to such a body?
Trying to approach this confusion intuitively, I'm imagining a fully enclosed metal pot of really hot water but with water that is not in motion (no convection) surrounded by a cooler body of water in an insulated metal pot. At some instant, the hot water in the inner pot has a temperature T_I and the hot cooler water in the outer pot has a temperature T_O. due to the temperature difference, I can use Fourier's equation to determine the rate of heat transfer by conduction from the hot water through the pot to the outside air. But where I'm confuse about the topic of conduction is when I consider how the energy of the pot with the energy balance equation. Heat has to flow into the pot then out of it into the air due to the temperature difference. At all times, I'd imagine that (T_O+T_I)/2 is constant and as the hot water cools and the cooler water warms until equilibrium is reached. So that means the metal pot separating the two bodies of water isn't changing in temp. In other words, for that pot, the rate of energy of the pot system separating the two bodies of water is equal to zero.
So going back to the original question, since the rate of energy change of the pot system is equal to zero and there is not heat generation, Q_dot.in - Q_dot.out=0. I also know that Q_dot.conduction=-kA(dT/dx) and heat conduction through some body has energy going in then energy out. So is it valid to say that Q_dot.conduction=Q_dot.in + Q_dot.out, e.g. with the two terms being or equal magnitude?
Fourier
Energy Balance
energy in - energy out = system energy change
rate of energy in - rate of energy out = rate of system energy change
Heat Conduction Equation
So is Q_dot.cond is the rate of heat transfer through a distance x by conduction due to a temperature difference. That means there is heat energy flowing from into the body from the higher temperature side and exiting through the lower temperature side. Does that mean Q_dot.cond = Q_dot.x + Q_dot.x+deltax (The first two terms of the Heat Conduction equation except they are summed) which is the sum of energy in one direction from high to low.
Asked another way, if I were considering heat conduction through some body, how is the energy balance equation applied to such a body?
Trying to approach this confusion intuitively, I'm imagining a fully enclosed metal pot of really hot water but with water that is not in motion (no convection) surrounded by a cooler body of water in an insulated metal pot. At some instant, the hot water in the inner pot has a temperature T_I and the hot cooler water in the outer pot has a temperature T_O. due to the temperature difference, I can use Fourier's equation to determine the rate of heat transfer by conduction from the hot water through the pot to the outside air. But where I'm confuse about the topic of conduction is when I consider how the energy of the pot with the energy balance equation. Heat has to flow into the pot then out of it into the air due to the temperature difference. At all times, I'd imagine that (T_O+T_I)/2 is constant and as the hot water cools and the cooler water warms until equilibrium is reached. So that means the metal pot separating the two bodies of water isn't changing in temp. In other words, for that pot, the rate of energy of the pot system separating the two bodies of water is equal to zero.
So going back to the original question, since the rate of energy change of the pot system is equal to zero and there is not heat generation, Q_dot.in - Q_dot.out=0. I also know that Q_dot.conduction=-kA(dT/dx) and heat conduction through some body has energy going in then energy out. So is it valid to say that Q_dot.conduction=Q_dot.in + Q_dot.out, e.g. with the two terms being or equal magnitude?
Last edited: