phoenixXL
- 49
- 4
Question
Find out the degree of unsaturation in a compound having the molecular formulae C9H6N4.
Attempt
The point lies in making the possible structure(s).
The structure that I felt possible is as follows
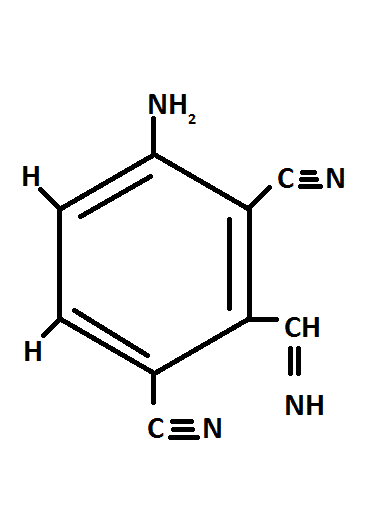
In the compound above there are 8-π electrons,
Hence the degree of unsaturation is 8(Ans)
Problem
The problem comes from the topic of structural isomerism and the book says the answer to be 9
Please help me out. Thanks for your time.
Find out the degree of unsaturation in a compound having the molecular formulae C9H6N4.
Attempt
The point lies in making the possible structure(s).
The structure that I felt possible is as follows
In the compound above there are 8-π electrons,
Hence the degree of unsaturation is 8(Ans)
Problem
The problem comes from the topic of structural isomerism and the book says the answer to be 9
Please help me out. Thanks for your time.