JoJoQuinoa
- 17
- 0
Hello,
I was wondering if someone could help clarifying this question.
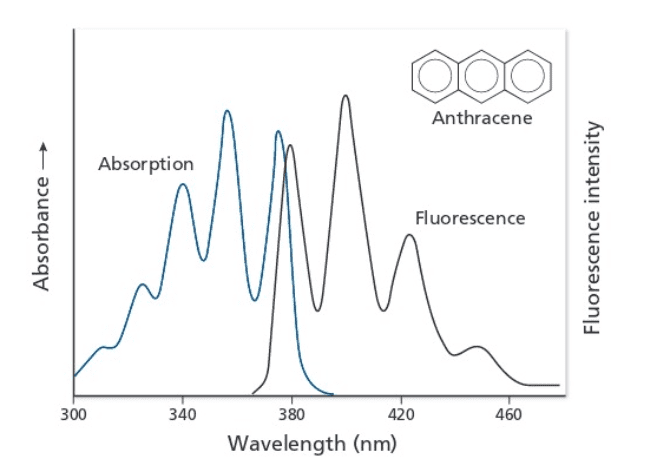
The question asks to estimate the energy state difference between the vibrational ground state of S0,v=0 and the first excited vibrational ground state S0,v=1 of the spectra below.
The given solution: S1,v=1 -> S0,v=1 at \lambda = 400 nm and S1,v=2 -> S0,v=1 at \lambda = 380 nm.
There are two things I'm confused about the solution:
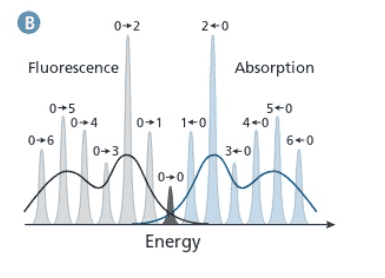
1) From Figure B, I would assume that the first Fluorescence peak at 380 nm in Figure A corresponds to S1,v=0 -> S0,v=1 and the second peak at 400 nm corresponds to S1,v=0 -> S0,v=2. Larger transition results in higher emission energy or smaller wavelength.
2) Why is S0,v=1 being used as the final state for both peaks?
The S1,v=1 -> S0,v=1 would occur as S1,v=1 ->S1,v=0 ->S0,v=1 and
S1,v=2 ->S1,v=0 ->S0,v=1.
Since S1,v=2 or 1 ->S1,v=0 is internal conversion, wouldn't S1,v=0 ->S0,v=1 for the two transitions give off the same energy?
Thanks in advance!
I was wondering if someone could help clarifying this question.
The question asks to estimate the energy state difference between the vibrational ground state of S0,v=0 and the first excited vibrational ground state S0,v=1 of the spectra below.
The given solution: S1,v=1 -> S0,v=1 at \lambda = 400 nm and S1,v=2 -> S0,v=1 at \lambda = 380 nm.
There are two things I'm confused about the solution:
1) From Figure B, I would assume that the first Fluorescence peak at 380 nm in Figure A corresponds to S1,v=0 -> S0,v=1 and the second peak at 400 nm corresponds to S1,v=0 -> S0,v=2. Larger transition results in higher emission energy or smaller wavelength.
2) Why is S0,v=1 being used as the final state for both peaks?
The S1,v=1 -> S0,v=1 would occur as S1,v=1 ->S1,v=0 ->S0,v=1 and
S1,v=2 ->S1,v=0 ->S0,v=1.
Since S1,v=2 or 1 ->S1,v=0 is internal conversion, wouldn't S1,v=0 ->S0,v=1 for the two transitions give off the same energy?
Thanks in advance!