rwooduk
- 757
- 59
- Homework Statement
- It's in a paper I am reading (not homework)
- Relevant Equations
- ppm = (mass of solute ÷ mass of solution x 1,000,000
I'm a little confused by a basic statement in a paper I am reading. It says that calcium chloride (1 M) and sodium carbonate (1 M) i.e. equimolar, have "a predetermined concentration of 3500 ppm of Ca2+"...
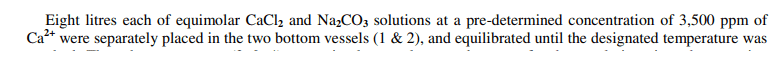
I understand that the mixing of these two will cause a precipitation reaction, but don't understand how the ppm of calcium ions was determined, was it measured / calculated somehow? Why would there be Ca2+ in the sodium carbonate solution? The statement seems a little confusing.
Thank you in advance for any help understanding this.
EDIT paper is Calcium carbonate scale formation in pipes: effect of flow rates, temperature, and malic acid as additives on the mass and morphology of the scale
I understand that the mixing of these two will cause a precipitation reaction, but don't understand how the ppm of calcium ions was determined, was it measured / calculated somehow? Why would there be Ca2+ in the sodium carbonate solution? The statement seems a little confusing.
Thank you in advance for any help understanding this.
EDIT paper is Calcium carbonate scale formation in pipes: effect of flow rates, temperature, and malic acid as additives on the mass and morphology of the scale