- #1
Unto
- 128
- 0
Hello, I'm doing an MSc project concerned with the treatment of plastic polymers with an μ-APPJ. I have been getting a tonne of results on different plastics using an APPJ of He carrier gas with an Oxygen admixture of 1/2, 1 & 2%.
However, in my analysis I'm unsure of what the dispersive component of the surface energy is and why it more or less stays the same.
Can someone give me a definition of what it is? I think its to do with van der waals but nothing on the net is giving me a good answer.
Is it supposed to stay the same irrespective of whether the material has been heated up or its polar component increases?
How would I interpretate my data on the surface energies for example:
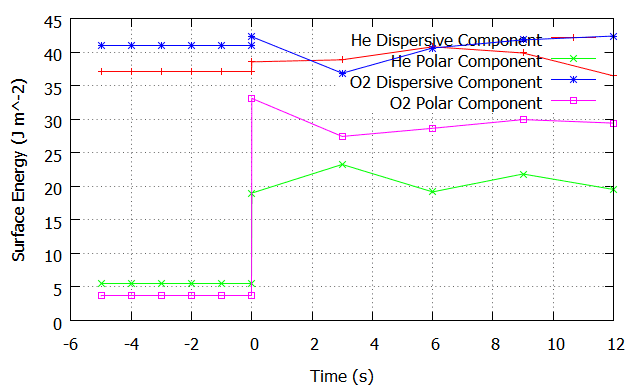
For convention, -5mins to 0mins is the 5minutes of plasma treatment. I'm not measuring the plastic during this time, hence the constant values and the discontinuity when I first measure it after treatment.
This is one of my preliminary results with Acetate plastic. Treated 1 run with He gas, 2nd run with He + O2. The legend will tell you. The dispersive components stay relatively the same, why?
However, in my analysis I'm unsure of what the dispersive component of the surface energy is and why it more or less stays the same.
Can someone give me a definition of what it is? I think its to do with van der waals but nothing on the net is giving me a good answer.
Is it supposed to stay the same irrespective of whether the material has been heated up or its polar component increases?
How would I interpretate my data on the surface energies for example:
For convention, -5mins to 0mins is the 5minutes of plasma treatment. I'm not measuring the plastic during this time, hence the constant values and the discontinuity when I first measure it after treatment.
This is one of my preliminary results with Acetate plastic. Treated 1 run with He gas, 2nd run with He + O2. The legend will tell you. The dispersive components stay relatively the same, why?