says
- 585
- 12
What does this diagram tell us about the distribution of nucleons in the nuclei? - The diagram is from Krane Introductory Nuclear Physics
I know that nucleons don't congregate around a central part of a nucleus, but instead have a constant distribution throughout. i.e. The number of nucleons per unit of volume is fairly constant.
I'm not really sure why they all taper off the way they do though. Smaller mass number particles tend to taper off quicker, while larger mass number particles taper off slower. I'm not really sure why this is.
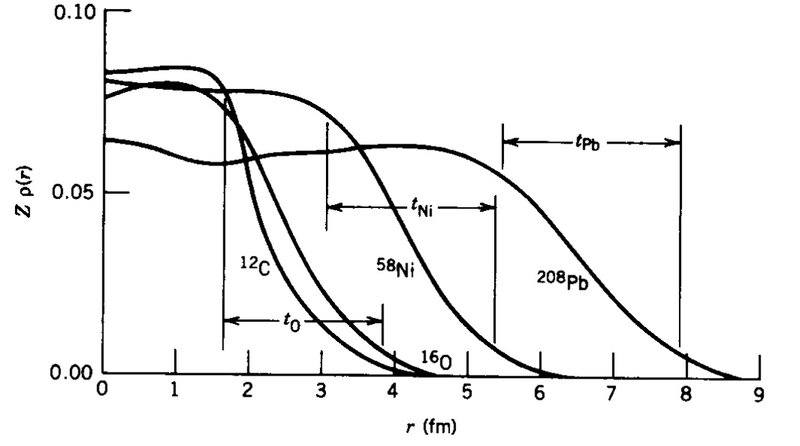
I know that nucleons don't congregate around a central part of a nucleus, but instead have a constant distribution throughout. i.e. The number of nucleons per unit of volume is fairly constant.
I'm not really sure why they all taper off the way they do though. Smaller mass number particles tend to taper off quicker, while larger mass number particles taper off slower. I'm not really sure why this is.
