SUMMARY
This discussion focuses on calculating nuclear reactions, specifically the fusion of isotopes such as deuterium into helium. Participants clarify that isotopes do not possess individual temperatures, as temperature is a collective property of a system. The Coulomb barrier, which represents the electric potential energy between two nuclei before they merge, is also discussed, along with the Gamow factor, which quantifies the likelihood of nuclear reactions occurring. Understanding these concepts is essential for anyone studying nuclear physics.
PREREQUISITES
- Basic understanding of nuclear reactions and fusion processes
- Familiarity with isotopes, particularly deuterium and helium
- Knowledge of the Coulomb barrier and its implications in nuclear physics
- Understanding of the Gamow factor in relation to nuclear reaction probabilities
NEXT STEPS
- Study the mathematical calculations involved in nuclear fusion reactions
- Research the properties and behaviors of isotopes in nuclear physics
- Learn about the Coulomb barrier and its role in nuclear fusion
- Explore the Gamow factor and its application in predicting nuclear reaction rates
USEFUL FOR
Students and enthusiasts of nuclear physics, researchers in nuclear energy, and anyone interested in the mechanics of atomic fusion and isotopic behavior.
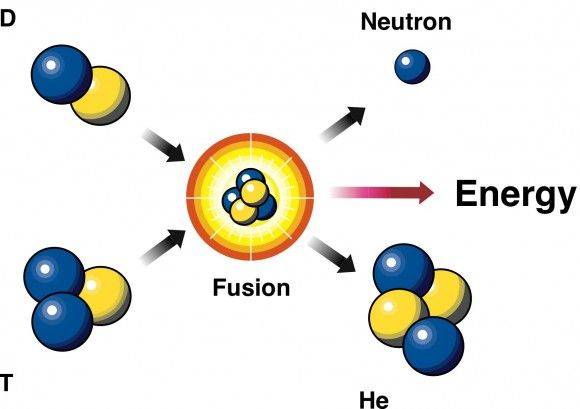
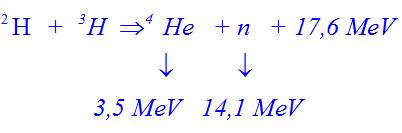 Can someone explain me the calculations? I'm new to the forum, please!
Can someone explain me the calculations? I'm new to the forum, please!