pamputt
- 9
- 0
Hi, is it true that the heavy atoms decaying only by electron capture should have globally a half-life shorter than ligher nuclei (decaying also only by electron capture)? This assumption comes from the fact heavy atoms have inner electron "closer" to their nucleus than the lighter ones and so a probability of finding electron inside the nucleus higher than for the ligher atoms (because their inner electrons are "further" from the nucleus).
If this is true, why is it not what we observe:
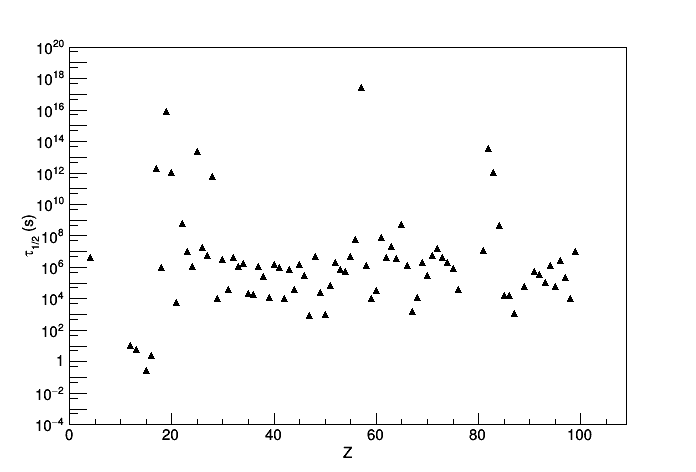
On this picture, I plotted the half-life of all the nuclei decaying only by electron capture as a function of their atomic number Z. Data come from NNDC. I just plotted naively th whole data so maybe there is a smarter way to do but basically I expected to see a general decrease of the half-life with respect to the Z. However, it looks rather flat.
Do you have any explanation?
If this is true, why is it not what we observe:
On this picture, I plotted the half-life of all the nuclei decaying only by electron capture as a function of their atomic number Z. Data come from NNDC. I just plotted naively th whole data so maybe there is a smarter way to do but basically I expected to see a general decrease of the half-life with respect to the Z. However, it looks rather flat.
Do you have any explanation?
Last edited by a moderator: