SUMMARY
The discussion centers on solving a dynamic equilibrium problem involving multiple reactions and the use of ICE (Initial, Change, Equilibrium) tables. Participants emphasize the importance of correctly identifying the reaction equations and using a single variable to represent changes in moles for accurate calculations. The consensus is that only one of the provided reaction choices will yield a total mole count of 2+x at equilibrium, highlighting the necessity of careful analysis of each reaction's stoichiometry. The problem originates from a Cambridge CIE AS Level Chemistry question, indicating its relevance to advanced chemistry students.
PREREQUISITES
- Understanding of dynamic equilibrium concepts in chemistry
- Proficiency in constructing ICE tables for chemical reactions
- Familiarity with stoichiometry and mole ratios
- Knowledge of equilibrium constants and their implications
NEXT STEPS
- Study the construction and application of ICE tables in various chemical reactions
- Explore the concept of equilibrium constants and their calculations
- Practice solving dynamic equilibrium problems from Cambridge CIE AS Level Chemistry
- Review stoichiometric principles and their application in chemical equations
USEFUL FOR
Chemistry students, particularly those preparing for Cambridge CIE AS Level exams, educators teaching equilibrium concepts, and anyone interested in mastering dynamic equilibrium problem-solving techniques.

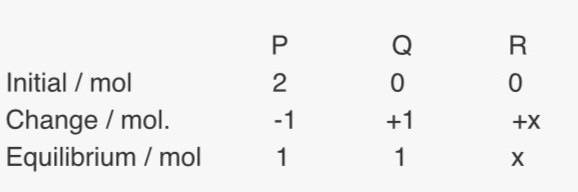 Since they said at equilibrium, the total no. of moles is (2+x), so P and Q should have 1 mol each at equilibrium. So P and Q are in 1:1 ratio, but how do they know x is 1 ? Ans is B
Since they said at equilibrium, the total no. of moles is (2+x), so P and Q should have 1 mol each at equilibrium. So P and Q are in 1:1 ratio, but how do they know x is 1 ? Ans is B you can see it immediately obvious. I haven't done this one long way or short way - but in this other recent problem
you can see it immediately obvious. I haven't done this one long way or short way - but in this other recent problem  this question is from Cambridge CIE AS level Chemistry.
this question is from Cambridge CIE AS level Chemistry. Oops silly me, I take it all back. For some reason I had been missing the first words of the question, that there were two moles to start with. Quite easy then.
Oops silly me, I take it all back. For some reason I had been missing the first words of the question, that there were two moles to start with. Quite easy then.