- #1
Janiceleong26
- 276
- 4
1. Homework Statement

This is what I did:
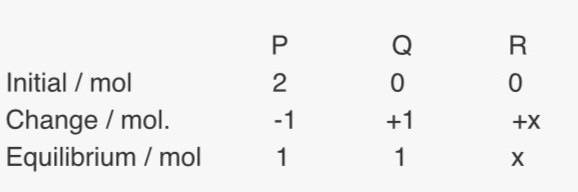 Since they said at equilibrium, the total no. of moles is (2+x), so P and Q should have 1 mol each at equilibrium. So P and Q are in 1:1 ratio, but how do they know x is 1 ? Ans is B
Since they said at equilibrium, the total no. of moles is (2+x), so P and Q should have 1 mol each at equilibrium. So P and Q are in 1:1 ratio, but how do they know x is 1 ? Ans is B
Homework Equations
The Attempt at a Solution
This is what I did:
 you can see it immediately obvious. I haven't done this one long way or short way - but in this other recent problem
you can see it immediately obvious. I haven't done this one long way or short way - but in this other recent problem  this question is from Cambridge CIE AS level Chemistry.
this question is from Cambridge CIE AS level Chemistry. Oops silly me, I take it all back. For some reason I had been missing the first words of the question, that there were two moles to start with. Quite easy then.
Oops silly me, I take it all back. For some reason I had been missing the first words of the question, that there were two moles to start with. Quite easy then.