Bolter
- 262
- 31
- Homework Statement
- See full question below
- Relevant Equations
- A = Lambda*N
Hi there
So I have had a go at this question but I'm not confident that I have done the last 2 parts of this question right?
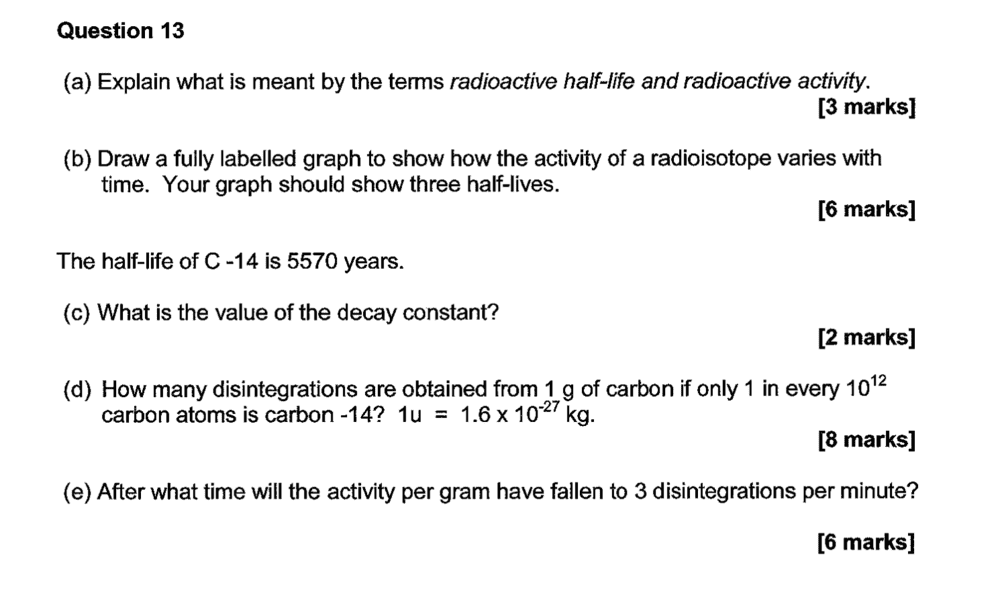
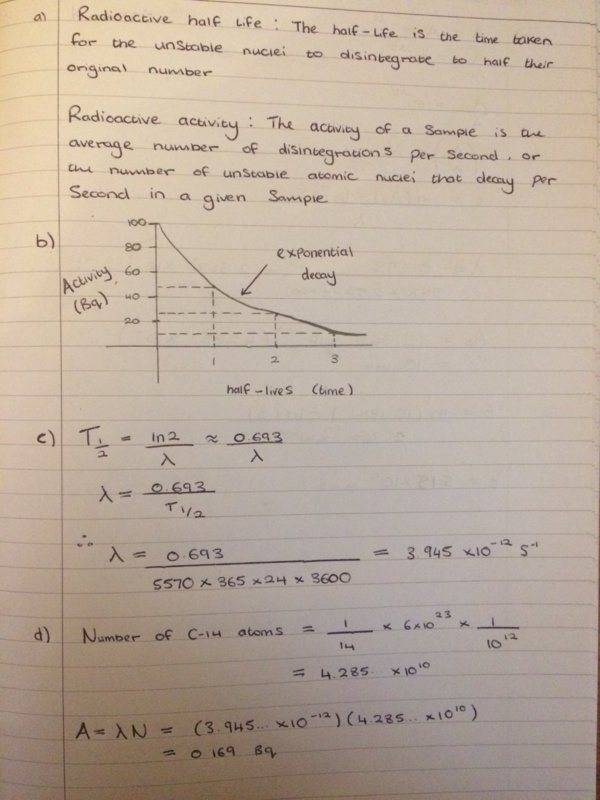
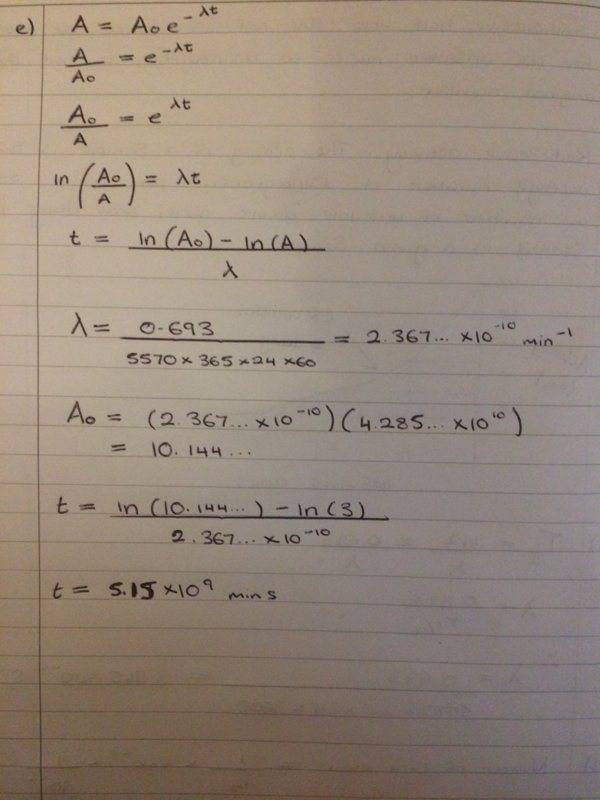
Can anyone please see if this is what you do to get the values?
Any help would be appreciated! Thanks
So I have had a go at this question but I'm not confident that I have done the last 2 parts of this question right?
Can anyone please see if this is what you do to get the values?
Any help would be appreciated! Thanks