WilliamL
- 1
- 0
Hi all,
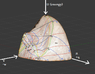
A few weeks ago I found http://www.sv.vt.edu/classes/ESM4714/methods/Gibbs.html" by Dr. Kriz of Virginia Tech. He describes how Gibbs envisioned a 3D U-S-V (energy-entropy-volume) surface, from which Maxwell created a sculpture.
Maxwell believed the surface was of great importance:
"Gibbs and Maxwell continued to use the graphical method to develop
the thermodynamic theory of state by asking the question:
What thermodynamic processes exist when moving from point A to point B on
the energy-entropy-volume diagram?" (Kriz)
I have been unable to find much information about this surface outside of the above website and the original papers of Gibbs/Maxwell.
I was hoping to discover thermodynamic relationships this surface may help visualize. I have created a 3D model of the surface (I can attach the .blend file when I get to the computer lab) that allows many different perspectives.
The relationships this graph provides:
Can anyone provide insight into this surface?
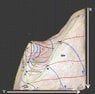
A few weeks ago I found http://www.sv.vt.edu/classes/ESM4714/methods/Gibbs.html" by Dr. Kriz of Virginia Tech. He describes how Gibbs envisioned a 3D U-S-V (energy-entropy-volume) surface, from which Maxwell created a sculpture.
Maxwell believed the surface was of great importance:
"Gibbs and Maxwell continued to use the graphical method to develop
the thermodynamic theory of state by asking the question:
What thermodynamic processes exist when moving from point A to point B on
the energy-entropy-volume diagram?" (Kriz)
I have been unable to find much information about this surface outside of the above website and the original papers of Gibbs/Maxwell.
I was hoping to discover thermodynamic relationships this surface may help visualize. I have created a 3D model of the surface (I can attach the .blend file when I get to the computer lab) that allows many different perspectives.
The relationships this graph provides:
- U-V-S, U-w-q
- Isotherms, Isobats, Isochores, Constant Entropy
- Phase changes
- Gibbs free energy (if you have a point below the surface, the Gibbs free energy is the vertical distance to the surface)
- Capacity for entropy (like above, only horizontal distance)
- and many more
Can anyone provide insight into this surface?
Attachments
Last edited by a moderator: