lily15
- 1
- 0
Hello, i have a few questions that i need help in if anyone can help me. I have tried to answer all of them, yet the answers i get the website i use to input the answers says it's wrong. Hopefully someone can help. Thanks.
1) A quantum system's lowest three allowed energies are as shown in the figure below. What possible energies of the n = 4 state would lead to an emission line at 360 nm?
Given:
alpha = 360 nm = 3.60*10E-7 m
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
e = 1.602*10E-19 C is elementary charge
Smallest energy:________eV *The answer i keep getting is 3.44eV but the site i am
using tells me it's wrong?
n3--------E3= 4.0eV
n2--------E2= 1.5eV
n1--------E1= 0.0eV
2) The measurements of a photoelectric-effect experiment are graphed in in the figure below, in which the intervals along the horizontal and vertical axes are respectively given by 4.0 1014 Hz and 1.49 V, respectively.
*From graph we get the f-axis intercept at Vstop = 0
*graph is attached
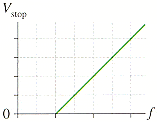
Given:
fmax = 4.0*10E14 Hz
Vmax = 1.49 V
e = 1.602*10E-19 C is elementary charge
h = 6.626*10E-34 J*s is Planck constant
a) What is the work function of the cathode?________eV * I keep getting 0.551eV and
again the site tells me it's wrong.
b)What experimental value of Planck's constant is obtained from these data?________J s
*Here i keep getting 8.95E-34 J*s
3)The figure below shows a molecular energy-level diagram. In the figure, E1 = 0.55 eV, E2 = 2.40 eV, and E3 = 3.00 eV.
*graph is attached
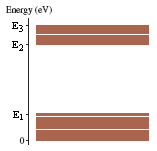
Given:
Eo = 0 eV
E1 = 0.55 eV
E2 = 2.40 eV
E3 = 3.00 eV
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
e = 1.602*10E-19 C is elementary charge
(a) What are the longest wavelength in the molecule's absorption spectrum?
Longest wavelength:________nm *The answer i get is 2250nm
(b) What are the longest and shortest wavelengths in the molecule's fluorescence spectrum?
Longest wavelength:________nm *the answer i get is 2250nm
Shortest wavelength:________nm *the answer i get is 413nm
4)The first three energy levels of the fictitious element X are shown in Figure P38.55, in which E1 = -3.7 eV, E2 = -1.7 eV, and E3 = -1.1 eV.
*graph is attached
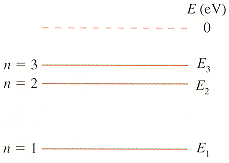
Given:
E1 = -3.7 eV
E2 = -1.7 eV
E3 = -1.1 eV
vo = 1.0*10E6 m/s
alpha = 2068 nm = 2.068*10-6 m
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
m = 9.109*10E-31 kg is mass of electron
(a) Calculate the (i) shortest and (ii) next-shortest wavelengths observed in the absorption spectrum of element X
(i)________nm *the answer i got was 335nm
(ii)________nm *the answer i got was 729nm
(d) An electron with a speed of 1.0 106 m/s collides with an atom of element X. Shortly afterward, the atom emits a 2068 nm photon. What was the electron's speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible. (Hint: The energy of the photon is not the energy transferred to the atom in the collision.)
________m/s *the answer i got was 8.88E5m/s
5) Fluorescence microscopy, discussed in a previous section, is an important tool in modern cell biology. A variation on this technique depends on a phenomenon known as two-photon excitation. If two photons are absorbed simultaneously (i.e., within about 10-16 s), their energies can add. A molecule that is normally excited by a 350 nm photon can be excited by two photons each having half as much energy. For this process to be useful, photons must illuminate the sample at the very high rate of at least 1029 photons/m2·s. This is achieved by focusing a laser beam to a small spot and by concentrating the power of the laser into very short (10-13 s) pulses that are fired 108 times each second. Suppose a biologist wants to use two-photon excitation to excite a molecular species that would be excited by 455 nm light in normal one-photon fluorescence microscopy. What minimum intensity must the laser beam have during each pulse?
Given:
alpha = 455 nm = 4.55*10-7 m
n = 10E29 photons/(m2•s)
delta t = 10E-13 s
N = 10E8 s-1
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
________W/m2 *the answer i got was 2.18E15 W/m2
1) A quantum system's lowest three allowed energies are as shown in the figure below. What possible energies of the n = 4 state would lead to an emission line at 360 nm?
Given:
alpha = 360 nm = 3.60*10E-7 m
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
e = 1.602*10E-19 C is elementary charge
Smallest energy:________eV *The answer i keep getting is 3.44eV but the site i am
using tells me it's wrong?
n3--------E3= 4.0eV
n2--------E2= 1.5eV
n1--------E1= 0.0eV
2) The measurements of a photoelectric-effect experiment are graphed in in the figure below, in which the intervals along the horizontal and vertical axes are respectively given by 4.0 1014 Hz and 1.49 V, respectively.
*From graph we get the f-axis intercept at Vstop = 0
*graph is attached
Given:
fmax = 4.0*10E14 Hz
Vmax = 1.49 V
e = 1.602*10E-19 C is elementary charge
h = 6.626*10E-34 J*s is Planck constant
a) What is the work function of the cathode?________eV * I keep getting 0.551eV and
again the site tells me it's wrong.
b)What experimental value of Planck's constant is obtained from these data?________J s
*Here i keep getting 8.95E-34 J*s
3)The figure below shows a molecular energy-level diagram. In the figure, E1 = 0.55 eV, E2 = 2.40 eV, and E3 = 3.00 eV.
*graph is attached
Given:
Eo = 0 eV
E1 = 0.55 eV
E2 = 2.40 eV
E3 = 3.00 eV
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
e = 1.602*10E-19 C is elementary charge
(a) What are the longest wavelength in the molecule's absorption spectrum?
Longest wavelength:________nm *The answer i get is 2250nm
(b) What are the longest and shortest wavelengths in the molecule's fluorescence spectrum?
Longest wavelength:________nm *the answer i get is 2250nm
Shortest wavelength:________nm *the answer i get is 413nm
4)The first three energy levels of the fictitious element X are shown in Figure P38.55, in which E1 = -3.7 eV, E2 = -1.7 eV, and E3 = -1.1 eV.
*graph is attached
Given:
E1 = -3.7 eV
E2 = -1.7 eV
E3 = -1.1 eV
vo = 1.0*10E6 m/s
alpha = 2068 nm = 2.068*10-6 m
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
m = 9.109*10E-31 kg is mass of electron
(a) Calculate the (i) shortest and (ii) next-shortest wavelengths observed in the absorption spectrum of element X
(i)________nm *the answer i got was 335nm
(ii)________nm *the answer i got was 729nm
(d) An electron with a speed of 1.0 106 m/s collides with an atom of element X. Shortly afterward, the atom emits a 2068 nm photon. What was the electron's speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible. (Hint: The energy of the photon is not the energy transferred to the atom in the collision.)
________m/s *the answer i got was 8.88E5m/s
5) Fluorescence microscopy, discussed in a previous section, is an important tool in modern cell biology. A variation on this technique depends on a phenomenon known as two-photon excitation. If two photons are absorbed simultaneously (i.e., within about 10-16 s), their energies can add. A molecule that is normally excited by a 350 nm photon can be excited by two photons each having half as much energy. For this process to be useful, photons must illuminate the sample at the very high rate of at least 1029 photons/m2·s. This is achieved by focusing a laser beam to a small spot and by concentrating the power of the laser into very short (10-13 s) pulses that are fired 108 times each second. Suppose a biologist wants to use two-photon excitation to excite a molecular species that would be excited by 455 nm light in normal one-photon fluorescence microscopy. What minimum intensity must the laser beam have during each pulse?
Given:
alpha = 455 nm = 4.55*10-7 m
n = 10E29 photons/(m2•s)
delta t = 10E-13 s
N = 10E8 s-1
c = 2.998*10E8 m/s is speed of light
h = 6.626*10E-34 J*s is Planck constant
________W/m2 *the answer i got was 2.18E15 W/m2
