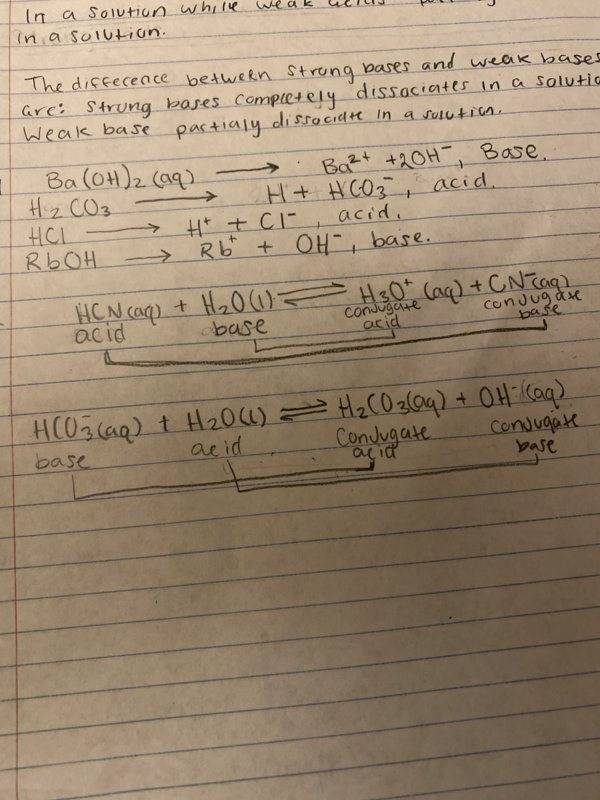Witcher
- 15
- 3
- Homework Statement
- I have a question that is more about why and less about how. If i am posting in the wrong section for my question i apologize ahead of time and ask that you direct me to the correct forum.
- Relevant Equations
- HCO3-(aq) + H2O(l) -> H2CO3(aq) + OH (aq)
<-
My question for Bronsted Lowry chemical equation is about the donating and accepting electrons. In the bottom chemical equation, the acid is a proton donor. How can hydrogen donate a proton when it has only 1? Or is the subscript the proton in chemical equations