- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How many Rb atoms to fit on a 9Cm^2 square?
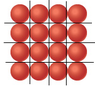
In summary, the conversation discusses a problem with calculating the number of atoms in a given area due to the empty spaces between the atoms. It is suggested to use the centers of the atoms and draw parallelograms instead of triangles. The conversation also mentions using a drawing program to create a diagram. Eventually, the problem is solved after a long time of thinking and studying.
Physics news on Phys.org
- #2
mfb
Mentor
- 37,125
- 13,967
There are empty spaces between the atoms - they need more space than just pi*r2.
- #3
UnD3R0aTh
- 90
- 0
exactly my thought, how to calculate that?!
- #4
mfb
Mentor
- 37,125
- 13,967
- #5
UnD3R0aTh
- 90
- 0
excellent, i solved the first one, but the second one I'm not really sure how to calculate the side of the triangle that's enclosing one atom!
- #6
mfb
Mentor
- 37,125
- 13,967
You can draw an equilateral triangle in the lattice.
- #7
UnD3R0aTh
- 90
- 0
yes but it looks like it's side is longer than the diameter, how to calculate it's length?!
- #8
UnD3R0aTh
- 90
- 0
no wait, the triangles do not work, they overlap! parallelograms work! how to calculate the area of a parallelogram using it's sides?
- #9
mfb
Mentor
- 37,125
- 13,967
Then you are drawing the wrong triangles. Use the centers of the atoms, this is easier. Afterwards, find out how many atoms per triangle (or triangles per atom) you have.
- #10
UnD3R0aTh
- 90
- 0
can u please draw a diagram like u did with the first one?
- #11
mfb
Mentor
- 37,125
- 13,967
Can you show your diagram?
- #12
UnD3R0aTh
- 90
- 0
i don't know to how to draw this, using what program?
- #13
mfb
Mentor
- 37,125
- 13,967
Every drawing program can draw lines. Windows always comes with Paint, for example.
- #14
UnD3R0aTh
- 90
- 0
pffffft I've been thinking about this problem for far too long I'm at my wits end, i really need to stop wasting my time with this and study, i feel nervous already cause i wasted so much time, please give me a more strong hint, save me some time, thank you
- #15
UnD3R0aTh
- 90
- 0
solved it
1. How do you calculate the number of Rb atoms that can fit on a 9Cm^2 square?
The number of atoms that can fit on a surface depends on the size of the atoms and the surface area. In this case, we can use the formula: Number of atoms = (Surface area)/(Atomic area). The atomic area of Rb is approximately 4.21 x 10^-20 cm^2. Therefore, the number of Rb atoms that can fit on a 9Cm^2 square would be 9Cm^2 / 4.21 x 10^-20 cm^2 = 2.14 x 10^20 atoms.
2. What is the atomic area of Rb?
The atomic area of Rb is approximately 4.21 x 10^-20 cm^2. This value is calculated by taking the square of the atomic radius, which is approximately 2.48 x 10^-8 cm.
3. Is the number of Rb atoms on a 9Cm^2 square the same as other surfaces?
No, the number of atoms that can fit on a surface depends on the size of the atoms and the surface area. Therefore, the number of Rb atoms that can fit on a 9Cm^2 square may be different on other surfaces with different areas.
4. Can you provide an example of a real-life application of calculating the number of atoms on a surface?
Sure, one example could be in the field of nanotechnology where scientists need to determine the number of atoms that can fit on a specific surface to create nanomaterials with desired properties.
5. What are some factors that can affect the accuracy of calculating the number of atoms on a surface?
The accuracy of calculating the number of atoms on a surface can be affected by factors such as the shape and size of the atoms, the surface roughness, and the experimental technique used to measure the surface area. Additionally, the temperature and pressure conditions can also play a role in the accuracy of the calculation.
Similar threads
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 622
-
Biology and Chemistry Homework Help
- Replies
- 11
- Views
- 16K
-
Atomic and Condensed Matter
- Replies
- 18
- Views
- 1K
-
Introductory Physics Homework Help
- Replies
- 2
- Views
- 1K
-
General Math
- Replies
- 1
- Views
- 2K
-
Biology and Chemistry Homework Help
- Replies
- 3
- Views
- 1K
-
Introductory Physics Homework Help
- Replies
- 1
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 2
- Views
- 11K
-
Science Fiction and Fantasy Media
- Replies
- 0
- Views
- 974
-
Introductory Physics Homework Help
- Replies
- 8
- Views
- 1K
Share: