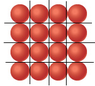
How many Rb atoms to fit on a 9Cm^2 square?
Click For Summary
SUMMARY
The discussion revolves around calculating the number of rubidium (Rb) atoms that can fit on a 9 cm² square. The radius of a single Rb atom is approximately 2.475 x 10^-8 cm, leading to a calculation that considers the area of the atom and the necessary spacing due to atomic structure. Participants debated the geometry involved, concluding that using parallelograms rather than triangles provides a more accurate representation of atomic packing. The final solution emphasizes the importance of accounting for empty spaces between atoms in the calculation.
PREREQUISITES- Basic chemistry knowledge
- Understanding of atomic radius and area calculations
- Familiarity with geometric shapes, specifically parallelograms
- Ability to use drawing software for visual representation
- Research atomic packing efficiency in solid-state chemistry
- Learn about geometric calculations involving parallelograms
- Explore software tools for scientific diagramming, such as GeoGebra
- Investigate the implications of atomic spacing in material science
Chemistry students, physicists, and material scientists interested in atomic structure and spatial calculations in solid materials.
Similar threads
- · Replies 1 ·
- · Replies 3 ·
Graduate
Probability of resonant ionization
- · Replies 18 ·
- · Replies 1 ·
- · Replies 2 ·
- · Replies 1 ·
- · Replies 5 ·