SUMMARY
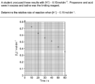
The discussion centers on converting iodine concentration ([I2]) to hydrogen ion concentration ([H+]) in a chemical reaction context. The user seeks to determine the time required for [H+] to reach 0.15 mol/L, given a graph of [I2] concentration over time. Participants suggest using a line of best fit to analyze the graph and emphasize the stoichiometric relationship between [I2] and [H+], noting that one mole of I2 produces a specific amount of H+. The importance of understanding reaction rates and the initial concentration of [H+] is also highlighted.
PREREQUISITES
- Understanding of stoichiometry in chemical reactions
- Familiarity with concentration units (mol/L)
- Ability to interpret graphs and perform linear regression analysis
- Knowledge of reaction kinetics and rates
NEXT STEPS
- Learn how to perform linear regression analysis on experimental data
- Study the stoichiometric relationships in chemical equations
- Explore the concept of reaction rates and their calculation
- Investigate the effects of buffering on pH and concentration changes
USEFUL FOR
Chemistry students, educators, and researchers involved in chemical kinetics and concentration analysis will benefit from this discussion.