Discussion Overview
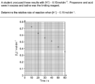
The discussion revolves around the conversion of iodine concentration ([I2]) to hydrogen ion concentration ([H+]) in the context of a chemical reaction. Participants are trying to determine how to calculate the time it takes for the [H+] concentration to reach 0.15 mol/L based on a provided graph of [I2] concentration over time.
Discussion Character
- Homework-related
- Technical explanation
- Debate/contested
Main Points Raised
- One participant expresses the need to convert [I2] concentration to [H+] concentration to find the time for [H+] to reach 0.15 mol/L.
- Another participant questions the necessity of converting [I2] to [H+] and seeks clarification on the original question.
- A participant suggests that the reaction rate appears constant and proposes using a line of best fit to analyze the data.
- There is mention of the chemical equation indicating the relationship between moles of [I2] and moles of [H+], suggesting that this could help in determining concentrations after a reaction.
- One participant speculates that the solution may have been buffered, implying that the pH remained constant throughout the reaction.
Areas of Agreement / Disagreement
Participants express differing levels of understanding regarding the problem and its requirements. There is no consensus on the best approach to convert concentrations or the relevance of certain assumptions about the reaction conditions.
Contextual Notes
Some participants express confusion about the initial conditions and the relationship between [I2] and [H+]. The discussion includes assumptions about reaction rates and the potential buffering of the solution, which may affect the interpretation of the data.