boladore
- 9
- 0
Hello. first, sorry for my poor English.
Derive characteristic wavelength from radiation powerThis is radiated power from bending magnet (synchrotron storage ring accelerating electron to get x-ray)

and these are wave distribution from storage ring




and what I have to derive is as follows. characteristic wavelength of radiation
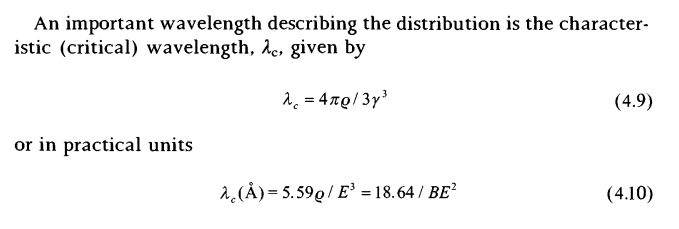 I have to derive this equation. professor said I can derive this equation from the first power equation. I have been trying for 3 days. but couldn't find solution.
I have to derive this equation. professor said I can derive this equation from the first power equation. I have been trying for 3 days. but couldn't find solution.
help...
Best regards.
Derive characteristic wavelength from radiation powerThis is radiated power from bending magnet (synchrotron storage ring accelerating electron to get x-ray)
and these are wave distribution from storage ring
and what I have to derive is as follows. characteristic wavelength of radiation
help...
Best regards.