SUMMARY
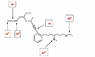
The discussion centers on the hybridization of a carbon atom depicted in an image, specifically questioning why it is labeled as sp instead of sp2 due to the presence of double bonds on both sides. A participant clarifies that the carbon in question indeed has two double bonds, which necessitates the use of two p orbitals for hybridization. This understanding aligns with the principles of hybridization in organic chemistry, confirming that the carbon's hybridization is correctly identified as sp.
PREREQUISITES
- Understanding of hybridization in organic chemistry
- Knowledge of carbon bonding and orbital theory
- Familiarity with double and triple bonds in molecular structures
- Basic grasp of molecular geometry and electron configuration
NEXT STEPS
- Study the concept of hybridization in greater detail, focusing on sp, sp2, and sp3 configurations
- Explore the role of p orbitals in bonding and hybridization
- Investigate examples of molecules with double and triple bonds to see hybridization in action
- Learn about molecular geometry and how it relates to hybridization types
USEFUL FOR
Chemistry students, educators, and anyone interested in understanding molecular hybridization and bonding in organic compounds.