fog37
- 1,566
- 108
- TL;DR
- understand energy levels in energy diagram for compounds
Hello,
I am trying to correctly interpret the energy diagram below.
For example, a diatomic molecule can translate (kinetic energy), rotate (rotational energy), vibrate (vibrational energy). Each different type of energy is quantized and has associated modes (also called states). The rotational, vibrational, translational energy states/levels/modes pertain to the molecule as a whole. On the other hand, electronic energy levels are electrostatic potential energy levels (not kinetic energy) and pertain to the electrons and nuclei.
The vertical axis in the diagram, labelled Energy, therefore indicates energy in general, correct?
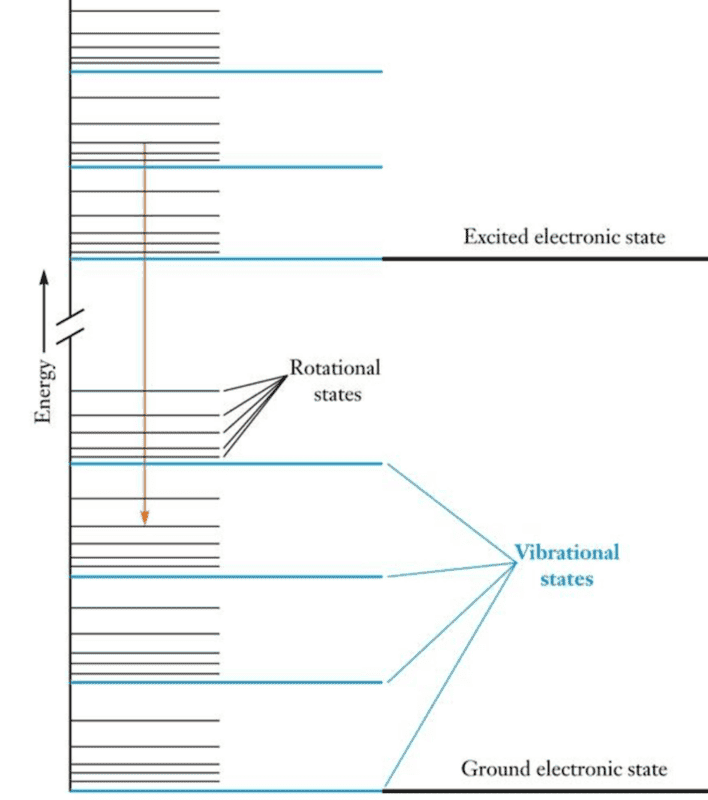 Based on the next figure below, what does it mean that an electronic energy level is comprised by several vibrational energy levels which are, in turn, comprised by many rotational energy levels? I know that when multiple molecules connect together, energy bands are formed instead of discrete and sharp energy levels. A band is formed by many discrete energy levels slightly separated from each other.
Based on the next figure below, what does it mean that an electronic energy level is comprised by several vibrational energy levels which are, in turn, comprised by many rotational energy levels? I know that when multiple molecules connect together, energy bands are formed instead of discrete and sharp energy levels. A band is formed by many discrete energy levels slightly separated from each other.
Do rotational/vibrational states get excited when a molecules absorbs external energy that does not match the difference between electronic energy levels?
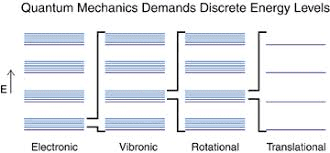
I am trying to correctly interpret the energy diagram below.
For example, a diatomic molecule can translate (kinetic energy), rotate (rotational energy), vibrate (vibrational energy). Each different type of energy is quantized and has associated modes (also called states). The rotational, vibrational, translational energy states/levels/modes pertain to the molecule as a whole. On the other hand, electronic energy levels are electrostatic potential energy levels (not kinetic energy) and pertain to the electrons and nuclei.
The vertical axis in the diagram, labelled Energy, therefore indicates energy in general, correct?
Do rotational/vibrational states get excited when a molecules absorbs external energy that does not match the difference between electronic energy levels?
Last edited: