pankaj66866
- 18
- 0
Hi everybody ,
This is the first time i am posting on this forum.
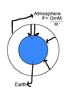
A couple of days back i was having a conversation with a religious person who went on telling that the position of Earth and gravity is so perfect that it was at right distance from sun and Earth is massive enough that oxygen is present as gas in the atmosphere ... so i wondered how massive the Earth should be that the atmosphere, under the influence of gravity compresses itself under its own weight such that oxygen is present as a liquid ... by calculating this we would know how much variation in Earth's mass is required for this to happen and hence determine whether this is divine plan or not.
Regards
Pankaj D
This is the first time i am posting on this forum.
A couple of days back i was having a conversation with a religious person who went on telling that the position of Earth and gravity is so perfect that it was at right distance from sun and Earth is massive enough that oxygen is present as gas in the atmosphere ... so i wondered how massive the Earth should be that the atmosphere, under the influence of gravity compresses itself under its own weight such that oxygen is present as a liquid ... by calculating this we would know how much variation in Earth's mass is required for this to happen and hence determine whether this is divine plan or not.
Regards
Pankaj D