SUMMARY
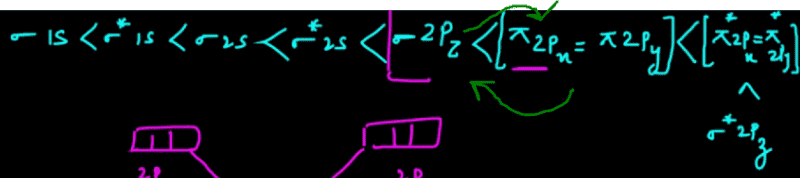
Molecular Orbital Theory (MOT) is essential for understanding the bonding in molecules, particularly for those with a higher electron count, such as BF3, which has 24 electrons. The discussion emphasizes the importance of knowing molecular geometry and symmetry, especially in the context of sp3 hybridization, where four orbitals form a tetrahedral arrangement. The user confirms the utility of MOT despite feeling overwhelmed by its complexity. Resources such as the Polyatomic Molecular Orbital Theory guide are recommended for further exploration.
PREREQUISITES
- Molecular Orbital Theory fundamentals
- Understanding of sp3 hybridization
- Basic knowledge of molecular geometry and symmetry
- Familiarity with bond order calculations
NEXT STEPS
- Study the Polyatomic Molecular Orbital Theory in detail
- Learn about bond order calculations for complex molecules
- Explore the implications of molecular geometry on bonding
- Investigate advanced concepts in molecular symmetry
USEFUL FOR
Chemistry students, educators, and professionals seeking to deepen their understanding of Molecular Orbital Theory and its applications in molecular bonding and geometry.