- #1
Adjax
- 13
- 0
This going to be a long post !
Orbital Dilemma:
i) Why there is opposite phase orbital for s-orbital? [ Couldn't find any representation on the web except for a pic in Ian Fleming's Molecular Orbitals and Organic Chemical Reactions,pg3)
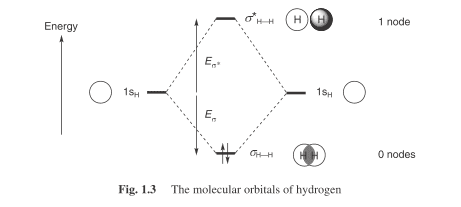
*I assert no right over the image,image is referenced under fair-use policy
ii) As shown in the image is it possible that or what is probability that in a given sample of Hydrogen atleast n hydrogens are out of phase and therefore doesn't react with other chemical species(can be hydrogen itself or any other chemical)
iii) Why the phenomenon of phase arises? Let's say two opposite phase orbitals makes anti-bonding orbital , the probability that electron is present is negligible or zero , the mathematical argument behind this also seems fine!
But why ?(what's physical intuition behind it), does electron field a kind of repulsion ? (Suppose I fire electron at such orbital with sufficiently low KE/negligible electron, will electron feel a repulsion there/what is preventin its presence there?)
iv) Can phase of the wavefunction be reverted? (in some scenario)
b)Ghosts of Hybrids
i)Lets start with carbonyl atom , the mayor of the world of organic reactions , I am new to the world organic reactions .In Clayden , the author says that a Nucelophile (CN) gives its electron to Electrophile CO's antibonding low energy LUMO π* ...<details of Mechanism skipped>
The thing which confuses me: The side-on combination of p-orbitals gives rise to π orbitals , as per the diagram of Carbonyl molecule both the left/unhybridized p orbital of sp2 hybridized atoms of C and O atom are in phase and side-on combo yields a π orbital...makes sense, but if both of the unhybridzed p-orbitals are in phase then how does the π* comes into the picture?
...Pausing for now as when I started writing questions I was 'in zone' and now due to certain circumstances I'm 'out of the zone'
Orbital Dilemma:
i) Why there is opposite phase orbital for s-orbital? [ Couldn't find any representation on the web except for a pic in Ian Fleming's Molecular Orbitals and Organic Chemical Reactions,pg3)
*I assert no right over the image,image is referenced under fair-use policy
ii) As shown in the image is it possible that or what is probability that in a given sample of Hydrogen atleast n hydrogens are out of phase and therefore doesn't react with other chemical species(can be hydrogen itself or any other chemical)
iii) Why the phenomenon of phase arises? Let's say two opposite phase orbitals makes anti-bonding orbital , the probability that electron is present is negligible or zero , the mathematical argument behind this also seems fine!
But why ?(what's physical intuition behind it), does electron field a kind of repulsion ? (Suppose I fire electron at such orbital with sufficiently low KE/negligible electron, will electron feel a repulsion there/what is preventin its presence there?)
iv) Can phase of the wavefunction be reverted? (in some scenario)
b)Ghosts of Hybrids
i)Lets start with carbonyl atom , the mayor of the world of organic reactions , I am new to the world organic reactions .In Clayden , the author says that a Nucelophile (CN) gives its electron to Electrophile CO's antibonding low energy LUMO π* ...<details of Mechanism skipped>
The thing which confuses me: The side-on combination of p-orbitals gives rise to π orbitals , as per the diagram of Carbonyl molecule both the left/unhybridized p orbital of sp2 hybridized atoms of C and O atom are in phase and side-on combo yields a π orbital...makes sense, but if both of the unhybridzed p-orbitals are in phase then how does the π* comes into the picture?
...Pausing for now as when I started writing questions I was 'in zone' and now due to certain circumstances I'm 'out of the zone'