SUMMARY
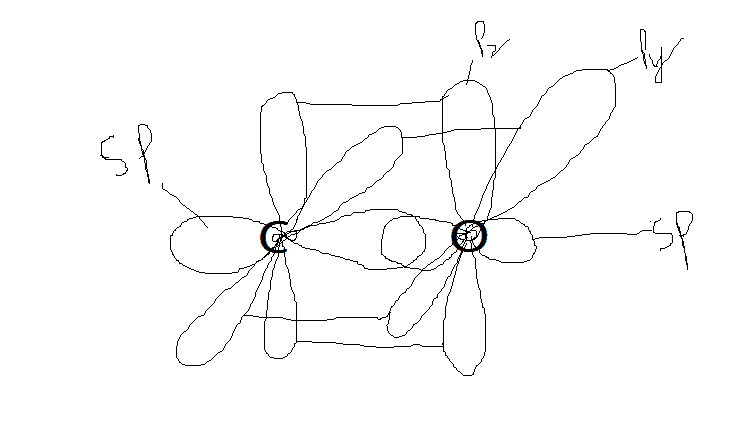
The forum discussion focuses on the hybridization and bonding structures of Carbon Monoxide (CO) and Carbon Dioxide (CO2). Users clarify that CO2's orbital diagrams are nearly accurate but require additional bonding representations for the negative phase of the Pz and Py orbitals. For CO, the resonance structures are discussed, emphasizing that the bond order cannot be distinctly represented in the diagrams. Additionally, the complexity of hybridization in larger atoms and the implications for bonding in compounds like PF3 and PF5 are highlighted.
PREREQUISITES
- Understanding of hybridization concepts in molecular chemistry
- Familiarity with resonance structures and bond order
- Knowledge of molecular orbital theory versus valence bond theory
- Basic principles of molecular geometry and bonding in CO and CO2
NEXT STEPS
- Research the differences between sp, sp2, and sp3 hybridization
- Learn about resonance structures in molecular bonding
- Study the implications of hybridization in larger atoms beyond the second period
- Explore the concept of banana bonds and their significance in molecular orbital theory
USEFUL FOR
Chemistry students, educators, and professionals interested in molecular bonding, hybridization, and resonance structures in small molecules like CO and CO2.