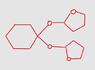
Organic chemistry acetal reaction help
- Thread starter IntegralDerivative
- Start date
Click For Summary
The discussion revolves around the acetal formation process, specifically involving cyclohexanol and carbonyl groups. Participants clarify that to create an acetal, one needs alcohols, a leaving group like water, and an acid catalyst. The conversation highlights the importance of understanding the hemiacetal intermediate, which is formed before the final acetal product. There is also a focus on the functionalities required for the reaction and the steps leading to the final product. Overall, the thread emphasizes the key components and mechanisms involved in acetal formation in organic chemistry.
Similar threads
- · Replies 2 ·
- · Replies 28 ·
- · Replies 7 ·
- · Replies 6 ·
Chemistry
Concentration of Phosphorus from KH2PHO4
- · Replies 4 ·
- · Replies 8 ·
- · Replies 4 ·
- · Replies 2 ·
Chemistry
Reactions With Dilute Hydrochloric Acid
- · Replies 3 ·