greg997
- 105
- 2
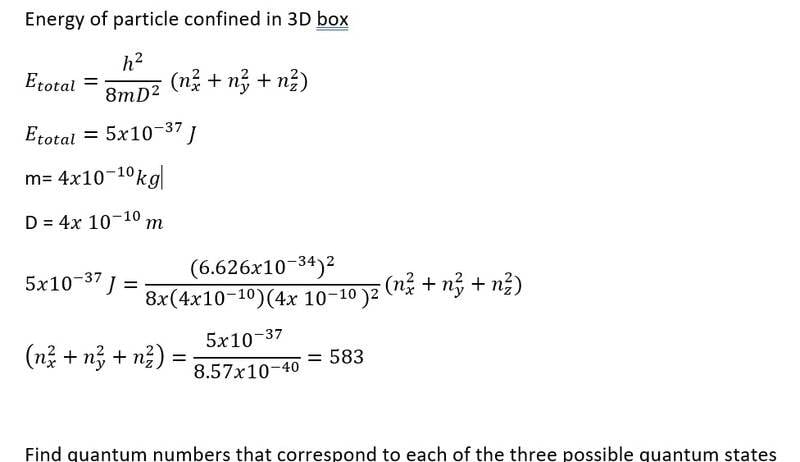
- Homework Statement
- Find quantum numbers for each of three possible quantum states
- Relevant Equations
- E= (( h^2)/(8mD) ) (nx^2_ny^2, nz^2)
Hi Everyone.
I hope someone can point me in right direction. I am struggling to work this out . If it was 1d confinement the calculated n number would be the energy level. So for example n= 3, means that quantum number is n= 3 and there is 3 possible quantum states. Is that correct?
With 3D box i am getting confused what values nx , ny, nx can have for the E given.
I hope someone can point me in right direction. I am struggling to work this out . If it was 1d confinement the calculated n number would be the energy level. So for example n= 3, means that quantum number is n= 3 and there is 3 possible quantum states. Is that correct?
With 3D box i am getting confused what values nx , ny, nx can have for the E given.