PainterGuy
- 938
- 73
Hi,
I was watching the following video.
Around 17:00 the following is said:
I tried to balance the chemical equation as presented in the video. Please have a look below. I don't see how the production of iron oxide yields more oxygen in the entire reaction. The heating of sodium chlorate produces three molecules of oxygen and these molecules are then used to oxidize iron. Where am I going wrong? Thanks, in advance!
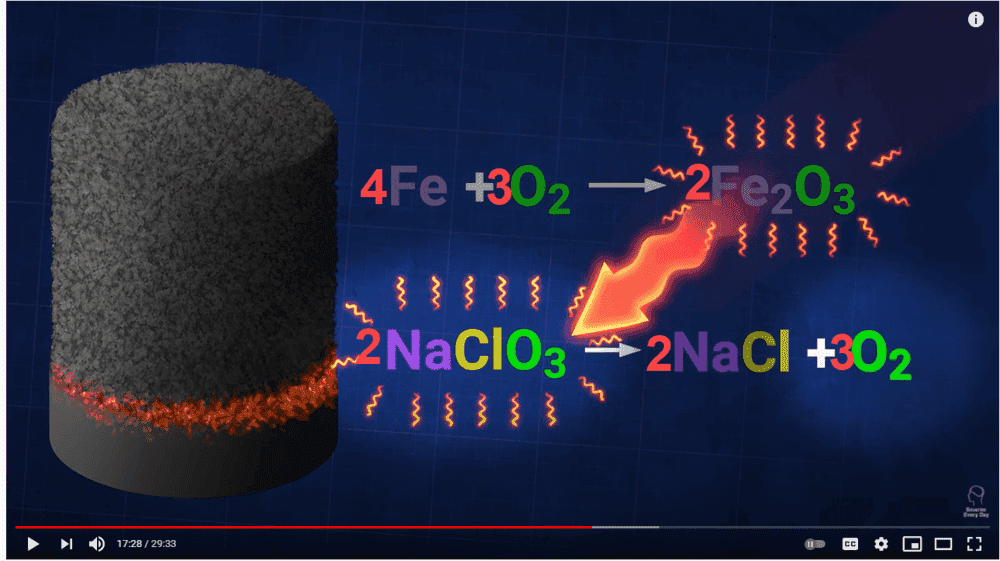
I was watching the following video.
Around 17:00 the following is said:
Okay. It wasn't until I got off the sub,
when I totally understood what was going on here.
But the two main chemicals in this candle were iron and sodium Chlorate.
When you burn iron,
that's adding oxygen to the iron and you're creating iron oxide.
You're actually creating heat. That's the burning of the candle.
But when you do that, there's also sodium chlorate in the candle.
And that heat from the iron oxide is liberating oxygen from the sodium chlorate.
and in doing so you actually get more oxygen from the chemical reaction.
I tried to balance the chemical equation as presented in the video. Please have a look below. I don't see how the production of iron oxide yields more oxygen in the entire reaction. The heating of sodium chlorate produces three molecules of oxygen and these molecules are then used to oxidize iron. Where am I going wrong? Thanks, in advance!
Last edited: