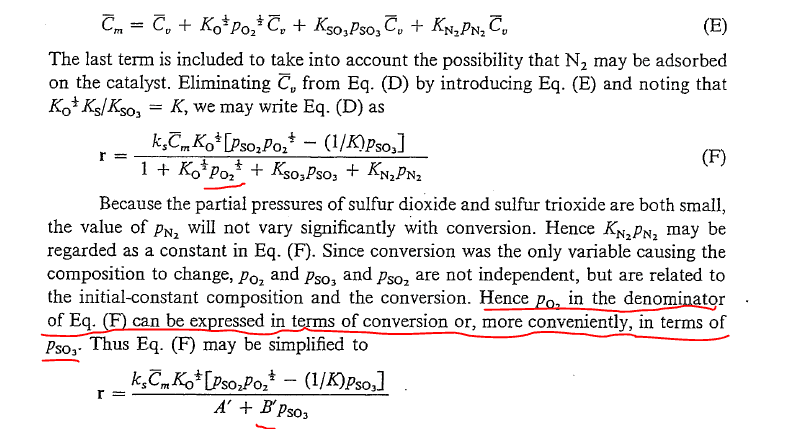
SUMMARY
The discussion centers on the modeling of catalysis, specifically addressing the relationship between the partial pressure of oxygen (O2) raised to the (1/2) power and its role in forming PSO3. Participants express frustration over the lack of clarity in the explanations provided in the referenced book, particularly regarding the approximation methods used in chemical reactions. The conversation highlights the challenges of expressing pressures between reactants and products when they are raised to different exponents, emphasizing the need for better pedagogical approaches in chemical modeling.
PREREQUISITES
- Understanding of chemical kinetics and catalysis
- Familiarity with partial pressure concepts in gas reactions
- Knowledge of reaction stoichiometry and equilibrium
- Basic proficiency in mathematical modeling of chemical reactions
NEXT STEPS
- Research the derivation of the rate laws in catalysis
- Study the impact of partial pressures on reaction rates using the Arrhenius equation
- Explore advanced chemical modeling software for reaction simulations
- Learn about the significance of exponents in reaction mechanisms and their approximations
USEFUL FOR
Chemistry students, researchers in catalysis, and professionals involved in chemical engineering or reaction modeling will benefit from this discussion.