SUMMARY
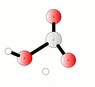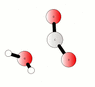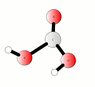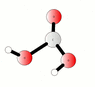
The discussion centers on the reaction between carbon dioxide (CO2) and water (H2O) to form carbonic acid (H2CO3), represented by the equilibrium equation CO2 + H2O <=> H2CO3. Participants clarify that while CO2 is weakly soluble in water, it exists in equilibrium with carbonic acid, and both species can interconvert rapidly. The conversation also highlights that carbonic acid can decompose back into CO2 and water, particularly in the presence of water, and discusses the implications of saturation and gas solubility in solutions.
PREREQUISITES
- Understanding of chemical equilibrium and reaction dynamics
- Familiarity with acid-base chemistry, specifically carbonic acid behavior
- Knowledge of gas solubility principles in liquids
- Basic grasp of quantum chemistry related to molecular stability
NEXT STEPS
- Research the solubility of gases in liquids, focusing on carbon dioxide and carbonic acid
- Study the principles of chemical equilibrium and how it applies to acid-base reactions
- Explore quantum calculations related to molecular stability and decomposition reactions
- Investigate methods for infusing high concentrations of CO2 in water for agricultural applications
USEFUL FOR
Chemists, environmental scientists, agricultural specialists, and anyone interested in the chemical behavior of carbon dioxide in aqueous solutions.