SUMMARY
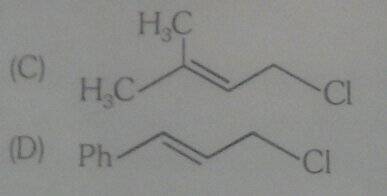
Compound C exhibits a higher rate of solvolysis than Compound D in a 50% aqueous ethanol solution at 45°C, primarily due to resonance effects. The solvent, a 50:50 ethanol and water mixture, enhances the solubility of the unsaturated halides involved in the reaction. The discussion highlights that the phenyl-substituted structure of Compound C destabilizes the carbon-chloride bond more effectively than Compound D, which lacks resonance. The reaction mechanism for these compounds is expected to follow an SN2 pathway due to the primary carbon attachment in Compound C.
PREREQUISITES
- Understanding of solvolysis reactions
- Knowledge of SN1 and SN2 reaction mechanisms
- Familiarity with resonance structures in organic chemistry
- Experience with solvent effects in chemical reactions
NEXT STEPS
- Research the impact of solvent polarity on reaction rates in organic chemistry
- Learn about the differences between SN1 and SN2 mechanisms in detail
- Explore the role of resonance in stabilizing reaction intermediates
- Investigate experimental methods for measuring reaction rates in solvolysis
USEFUL FOR
Chemistry students, organic chemists, and researchers interested in reaction mechanisms and solvolysis kinetics will benefit from this discussion.