Physics lover
- 249
- 25
- Homework Statement
- Is cyclopropyl cyclopentane carbocation also exceptionally stable or not?Give reason for you answer.
- Relevant Equations
- N/A
Here is an image of the structure
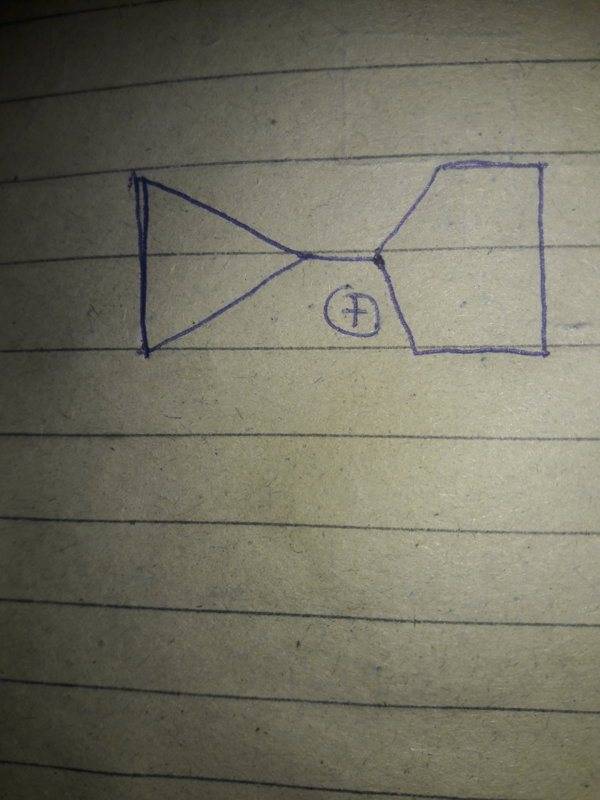
I know that cyclopropyl methyl carbocation is exceptionally stable due to an effect so called dancing resonance which takes place because of lot of strain in cyclopropyl ring and vacant p orbital of Carbon attached with the ring.
So I think this is a similar situation as vacant p orbital of carbon which has positive charge attached with cyclopropyl ring has its vacant p orbital perpendicular to bonding orbital.So dancing resonance can take place here.So it should also be exceptionally stable.I don't know the answer.Am I correct?
I know that cyclopropyl methyl carbocation is exceptionally stable due to an effect so called dancing resonance which takes place because of lot of strain in cyclopropyl ring and vacant p orbital of Carbon attached with the ring.
So I think this is a similar situation as vacant p orbital of carbon which has positive charge attached with cyclopropyl ring has its vacant p orbital perpendicular to bonding orbital.So dancing resonance can take place here.So it should also be exceptionally stable.I don't know the answer.Am I correct?