DarkEnergy890
- 14
- 3
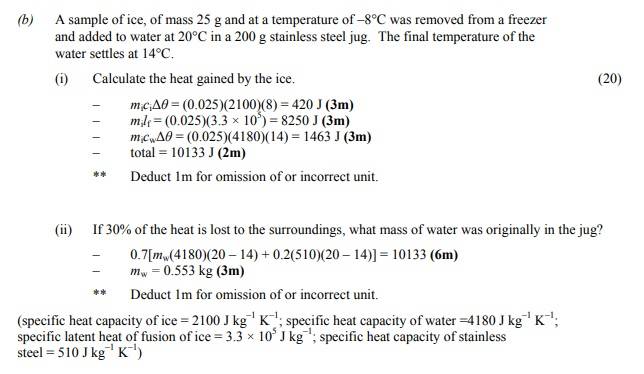
- Homework Statement
- https://prnt.sc/hq2fs_81J1vp
- Relevant Equations
- mc(deltatheta)
ml
During the procedure, 30% of heat is lost. So that means that 70% of water+container is contributing to melting the ice, right? And the other 30% contributing melting the ice is down to, well, the "heat being lost to the surroundings" (not sure what this really means).
We compute the sum and the mass is 0.553kg. Then I try to calculate what would happen if no heat was lost to the surroundings. The total energy would be approx 14000J! My question is that how can heat be lost to the surroundings? If anything, surely heat is being gained by the ice from the surroundings.
The only way I can think of heat being lost to the surroundings is if the room temperature was below 0 degrees, however this doesn't make sense because the final temperature of the water is 14 degrees.
[Mentor Note: Image from web link has been attached to the post]
Many thanks!
We compute the sum and the mass is 0.553kg. Then I try to calculate what would happen if no heat was lost to the surroundings. The total energy would be approx 14000J! My question is that how can heat be lost to the surroundings? If anything, surely heat is being gained by the ice from the surroundings.
The only way I can think of heat being lost to the surroundings is if the room temperature was below 0 degrees, however this doesn't make sense because the final temperature of the water is 14 degrees.
[Mentor Note: Image from web link has been attached to the post]
Many thanks!
Last edited by a moderator: