pks
I am trying to calculate how much a foil liner helps keep the inside of a package cool. I have calculated the rate of conduction but am now concerned with radiation.
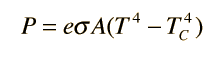
Above is the equation I am using. The emissivity for the material is 0.05, the area is 1 m^2, the outside air temp is 295 degree K, the air temp inside the package is 280 degrees K. This gives a value of 4 watts. Is using the air temp an over simplification or should I be using the outside surface temp of the package? I am trying to create a mathematical model that is as close as possible. Is there anything else I should be looking at?
Thanks in advance.
Above is the equation I am using. The emissivity for the material is 0.05, the area is 1 m^2, the outside air temp is 295 degree K, the air temp inside the package is 280 degrees K. This gives a value of 4 watts. Is using the air temp an over simplification or should I be using the outside surface temp of the package? I am trying to create a mathematical model that is as close as possible. Is there anything else I should be looking at?
Thanks in advance.
Last edited by a moderator: