butterchimkin
- 3
- 0
- TL;DR
- Safety advice for handling nitric acid.
Hello everyone. I’m a motorcycle mechanic working on a carburetor, and I was looking for advice.
To see pictures, scroll down.
I recently was working on a motorcycle, and there is a very fine brass needle called a fuel/air mixture screw that was tightened too much, and lodged in a tight airway.
The carburetor is made of aluminum. The airway is very tight, and there have been many mechanical solutions that I have tried, and none worked. I saw a solution that someone had using nitric acid to dissolve it on a motorcycle forum, and it worked for him.
I was wondering what are the safety procedures to doing something like this. How do I handle the acid, and how do I dispose of it once it’s done with?
Below I’ll attach links to the forum, and to the nitric acid that I ordered. My experience with chemistry is limited to a couple of classes in college.
Here are pictures of the needle tip that broke off, and the passage that it’s in (which decreases in diameter about half).

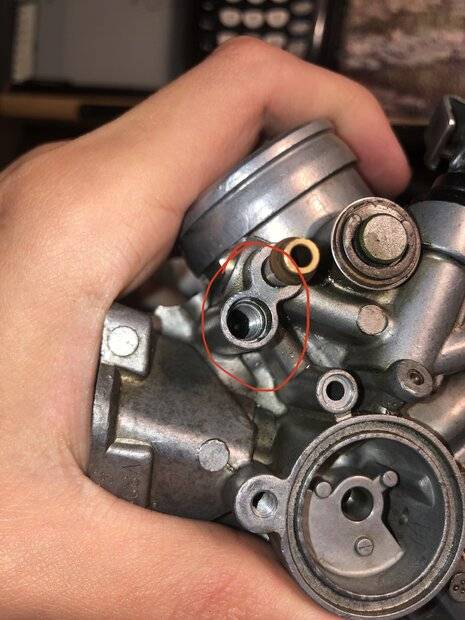
Here is the other side of the hole, which has a hard right turn into the brass needle about a quarter of an inch down.

Any advice is appreciated, thank you.
To see pictures, scroll down.
I recently was working on a motorcycle, and there is a very fine brass needle called a fuel/air mixture screw that was tightened too much, and lodged in a tight airway.
The carburetor is made of aluminum. The airway is very tight, and there have been many mechanical solutions that I have tried, and none worked. I saw a solution that someone had using nitric acid to dissolve it on a motorcycle forum, and it worked for him.
I was wondering what are the safety procedures to doing something like this. How do I handle the acid, and how do I dispose of it once it’s done with?
Below I’ll attach links to the forum, and to the nitric acid that I ordered. My experience with chemistry is limited to a couple of classes in college.
Here are pictures of the needle tip that broke off, and the passage that it’s in (which decreases in diameter about half).
Here is the other side of the hole, which has a hard right turn into the brass needle about a quarter of an inch down.
Any advice is appreciated, thank you.