aname
- 8
- 0
- Homework Statement
- i'm not sure I used the numbers right
- Relevant Equations
- Determination of the dissolution enthalpy
-(7.455 so,94+8,4) Delta T
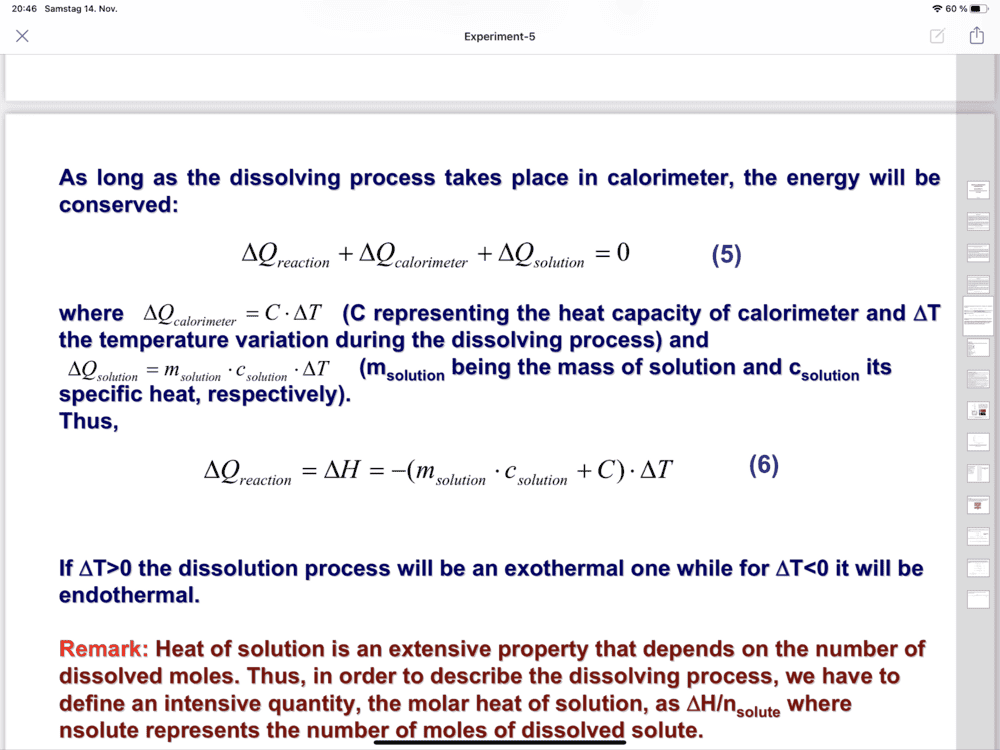
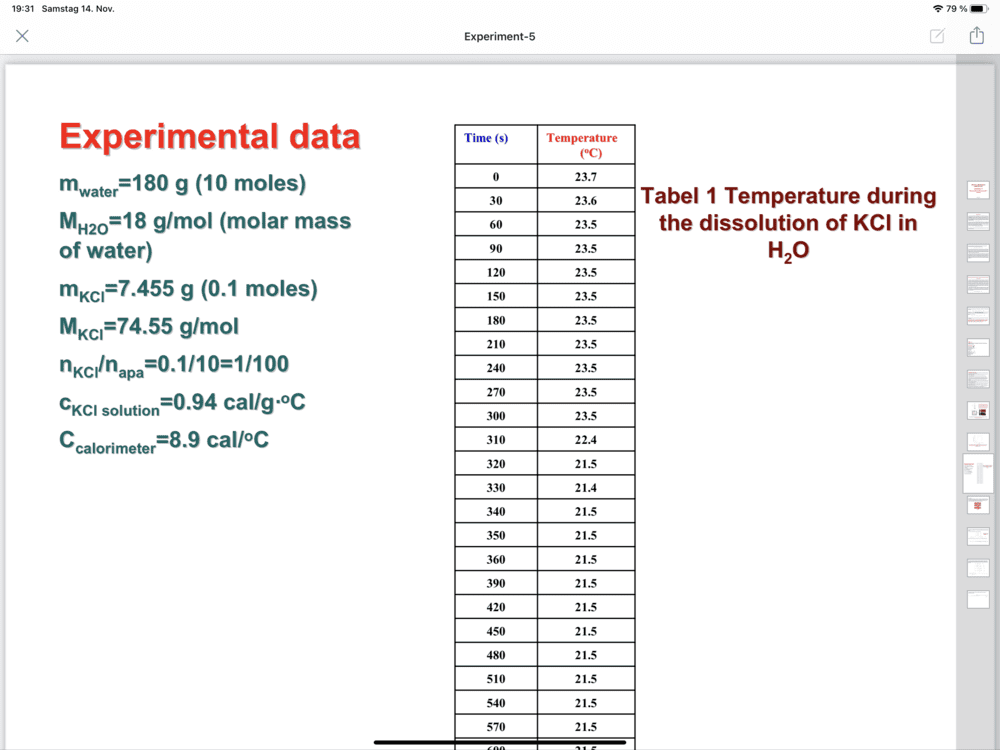
The discussion centers on the calculation of ΔH using the formula ΔH = (msolution x csolution + C) ΔT. The values provided include msolution = 7.455 g, Csolution = 0.94 J/g°C, and C = 8.9 J/g°C. The correct total mass of the solution is established as 187.5 g, derived from 180 g of water and 7.5 g of KCl. This clarification is crucial for accurate ΔH calculations.
PREREQUISITESChemistry students, laboratory technicians, and professionals involved in thermodynamic calculations and calorimetry. This discussion is particularly beneficial for those learning to calculate enthalpy changes in chemical solutions.
Hi,Chestermiller said:No. m of the solution is 187.5 g.