utsav55
- 14
- 0
What will be the % of Enol form of this compound?
99% or 1%? Also give reason.
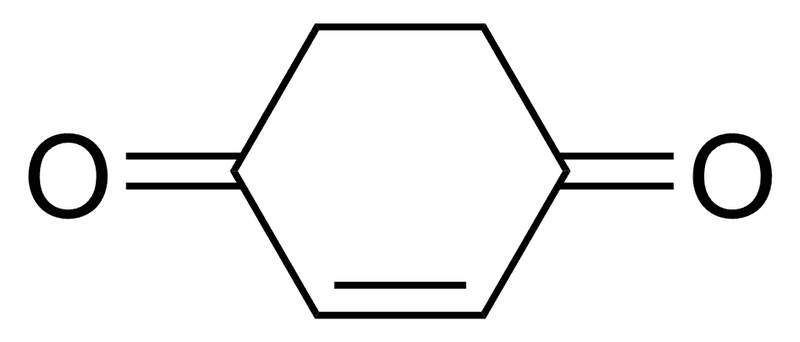
Thanks
99% or 1%? Also give reason.
Thanks
The discussion centers on determining the percentage of the enol form of a compound, with participants debating between 1% and 99%. The consensus leans towards 99% due to the stability provided by aromaticity in the enol form. The relationship between Gibbs free energy and chemical equilibrium is highlighted, emphasizing that small energy differences can significantly affect the composition of tautomeric forms. Aromatic stabilization energies for benzene, estimated between 20 to 40 kcal/mol, further support the predominance of the enol form.
PREREQUISITESChemistry students, organic chemists, and anyone interested in understanding tautomerism and the stability of chemical compounds.