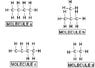
Which Structure Has The Highest Melting Point?
- Thread starter ProblemSets
- Start date
Click For Summary
SUMMARY
The discussion centers on determining which molecular structure has the highest melting point, focusing on the influence of intermolecular forces. Participants identify hydrogen bonding, dipole-dipole interactions, and London dispersion forces as key factors. The consensus emerges that structure A, which likely exhibits stronger intermolecular forces, is the correct answer, contrary to initial assumptions about structure C (1-butene). The conversation emphasizes the importance of understanding molecular features and their impact on melting points.
PREREQUISITES- Understanding of intermolecular forces: hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
- Basic knowledge of molecular structures and their properties.
- Familiarity with the concept of melting points in chemistry.
- Ability to analyze and compare molecular features.
- Research the characteristics of hydrogen bonding and its effect on melting points.
- Explore the differences between dipole-dipole interactions and London dispersion forces.
- Study the molecular structure of various hydrocarbons and their melting points.
- Learn about the role of molecular polarity in determining physical properties.
Chemistry students, educators, and anyone interested in molecular chemistry and the factors influencing melting points.
Similar threads
Undergrad
Melting point of graphite and diamond?
- · Replies 3 ·
- · Replies 5 ·
- · Replies 15 ·
- · Replies 9 ·
- · Replies 5 ·
- · Replies 5 ·
- · Replies 1 ·