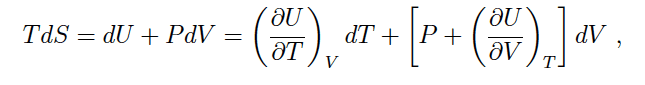Discussion Overview
The discussion revolves around the mathematical treatment of internal energy (U) in thermodynamics, specifically how it can be expressed in terms of temperature (T) and volume (V). Participants explore the implications of this expansion and its mathematical validity, as well as related concepts in thermodynamic properties.
Discussion Character
- Exploratory, Technical explanation, Conceptual clarification
Main Points Raised
- One participant questions whether there is a mathematical rule that permits the expansion of dU in terms of dT and dV, seeking clarification on the implications of this expansion.
- Another participant reiterates the question about the mathematical rule and emphasizes the expression of the differential of U with respect to T and V, suggesting a relationship between these variables.
- A separate query is raised regarding the number of intensive properties needed to define the state of a single phase material of constant composition, indicating a potential connection to the broader discussion of state variables.
Areas of Agreement / Disagreement
The discussion includes multiple viewpoints and questions, with no clear consensus reached on the mathematical rules governing the expansion of U or the requirements for specifying the state of a material.
Contextual Notes
Participants have not fully resolved the assumptions underlying the expansion of dU, nor have they clarified the definitions of intensive properties in the context of the discussion.