leopard
- 123
- 0
Why is a) more acidic than b) when C is more electron withdrawing than H? Electron withdrawal by a substituent increases acidity...
The discussion centers on the influence of electron-withdrawing and electron-donating groups on the acidity of organic compounds. It establishes that substituents like CH3, which are electron-donating, decrease acidity compared to electron-withdrawing groups. The comparison between compounds a) and b) illustrates that despite C being more electron-withdrawing than H, the presence of the CH3 group in b) reduces acidity due to its electron-donating nature. This highlights the critical role of substituent effects in determining acidity in organic chemistry.
PREREQUISITESChemistry students, organic chemists, and anyone interested in understanding the factors influencing acidity in organic compounds.
leopard said: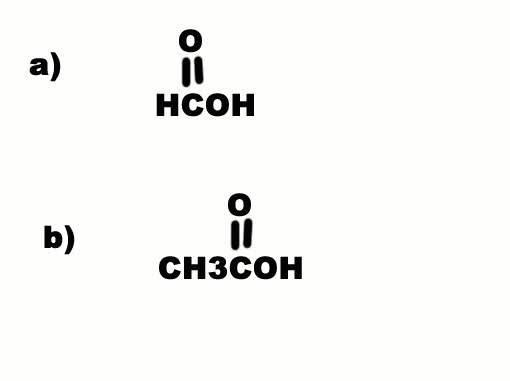
Why is a) more acidic than b) when C is more electron withdrawing than H? Electron withdrawal by a substituent increases acidity...