Hrnmhmm
- 2
- 0
My level of knowledge on Chemistry is first year. I'm teaching myself about electron configurations using Google to find and compare sources.
In this webpage - http://www.chemguide.co.uk/atoms/properties/ies.htm
It's demonstrated that Oxygen has a lower first ionization energy than Nitrogen despite having more protons. The explanation given is that the 2px suborbital in Oxygen (2px2) contains one more electron than in Nitrogen (2px1), forming an electron pair, and their repulsion negates some positive charge.
If the only difference between Nitrogen and Oxygen is in the electron pair 2px, why do Fluorine and Neon continue the second period's upward trend of ionization energy as normal? Shouldn't the repulsion of the additional electron pairs 2py2 and 2pz2 also affect ionization energy in these elements, reversing the trend?
In this webpage - http://www.chemguide.co.uk/atoms/properties/ies.htm
Jim Clark said: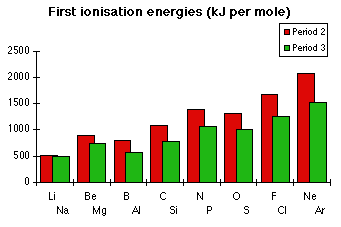
N ... 1s2, 2s2, 2px1, 2py1, 2pz1 ... 1st I.E. = 1400 kJ/mol
O ... 1s2, 2s2, 2px2, 2py1, 2pz1 ... 1st I.E. = 1310 kJ/mol
It's demonstrated that Oxygen has a lower first ionization energy than Nitrogen despite having more protons. The explanation given is that the 2px suborbital in Oxygen (2px2) contains one more electron than in Nitrogen (2px1), forming an electron pair, and their repulsion negates some positive charge.
If the only difference between Nitrogen and Oxygen is in the electron pair 2px, why do Fluorine and Neon continue the second period's upward trend of ionization energy as normal? Shouldn't the repulsion of the additional electron pairs 2py2 and 2pz2 also affect ionization energy in these elements, reversing the trend?
Last edited by a moderator: