diogo_sg
Hello. I'm new to this forum and to Physics and Chemistry in general and I have a question that's making me go crazy: why does the potential energy decrease as two atoms (say, hydrogen atoms) get closer to form a molecule? I'm talking about this graphic:
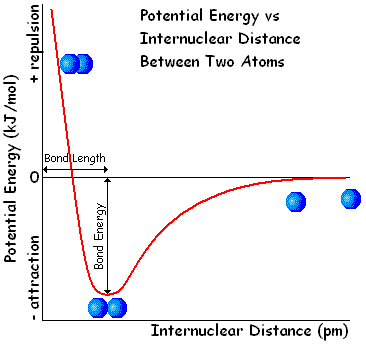
I've read that it's related to the increase of the atoms' kinetic energy, but I'm having a hard time figuring out how and why that happens.
Also, why does the potential energy get negative values and why does it increase again as it passes a certain value (4,52 eV in hydrogen's case)?
I understand that the potential energy changes with the distance between the atoms but I'd like to know why it changes the way it does. And please explain this as good as you can, using whatever Maths you need. I just want to understand this whole situation.
Thank you very much. Diogo
I've read that it's related to the increase of the atoms' kinetic energy, but I'm having a hard time figuring out how and why that happens.
Also, why does the potential energy get negative values and why does it increase again as it passes a certain value (4,52 eV in hydrogen's case)?
I understand that the potential energy changes with the distance between the atoms but I'd like to know why it changes the way it does. And please explain this as good as you can, using whatever Maths you need. I just want to understand this whole situation.
Thank you very much. Diogo