SUMMARY
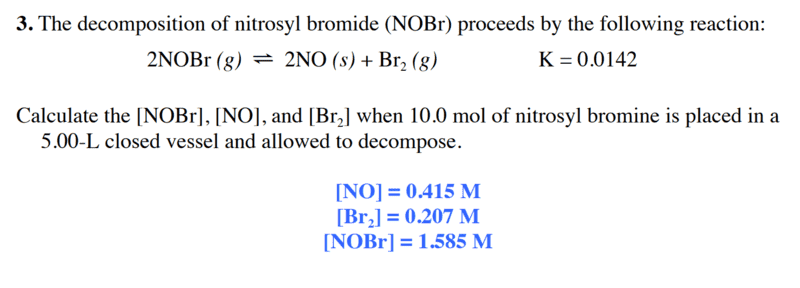
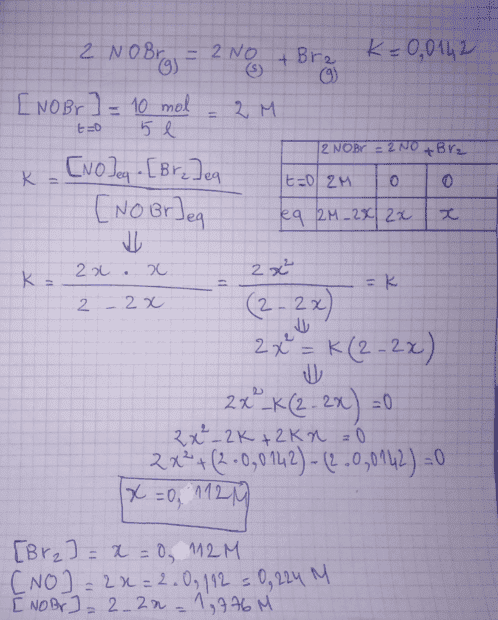
The forum discussion centers on a problem set regarding chemical equilibrium, specifically the equilibrium constant expression for the reaction involving nitrogen monoxide (NO), bromine (Br2), and nitrosyl bromide (NOBr). The user identifies a mistake in their calculation, noting the need to exponentiate the concentrations in the equilibrium expression: k = [NO]2[Br2]/[NOBr]2. Additionally, there is confusion regarding the physical state of NO, which is incorrectly listed as a solid under conditions that are not feasible, given its boiling point.
PREREQUISITES
- Understanding of chemical equilibrium concepts
- Familiarity with equilibrium constant expressions
- Knowledge of the physical states of substances at various temperatures
- Basic skills in stoichiometry and chemical reactions
NEXT STEPS
- Review the principles of chemical equilibrium and the derivation of equilibrium constant expressions
- Study the physical properties of nitrogen monoxide (NO) and its behavior at different temperatures
- Learn about the implications of temperature on the states of chemical substances
- Explore common mistakes in calculating equilibrium constants and how to avoid them
USEFUL FOR
Chemistry students, educators, and anyone involved in studying or teaching chemical equilibrium concepts.