Discussion Overview
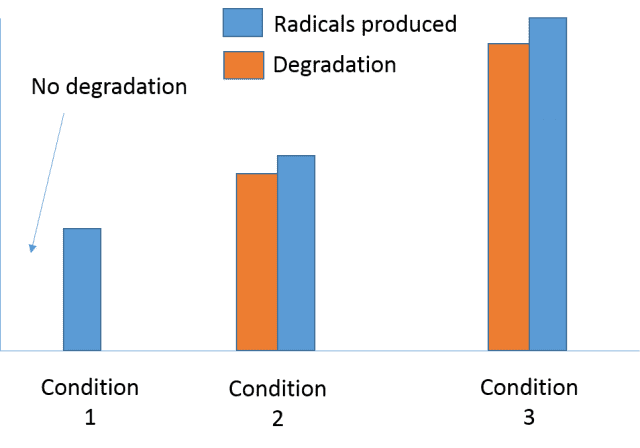
The discussion revolves around the relationship between radical formation and the degradation of pollutants during oxidation processes. Participants explore whether a minimum threshold of radicals is necessary for effective pollutant degradation and how varying conditions might influence this relationship.
Discussion Character
- Exploratory
- Debate/contested
- Technical explanation
Main Points Raised
- The original poster (OP) observes that pollutant degradation correlates with radical formation but notes instances where fewer radicals result in no degradation, raising questions about the necessity of a certain number of radicals.
- Some participants highlight the complexity of defining pollutants and radicals, suggesting that the diversity of molecules involved complicates the discussion.
- A participant uses an analogy comparing radicals to children attacking a group, proposing that a threshold number of radicals may be needed to initiate degradation, after which the process could accelerate.
- Another participant questions whether a model exists that describes a minimum threshold concentration of radicals required for reactions to begin.
- Some participants express that the OP lacks sufficient information for a thorough analysis, pointing out missing details such as the labeling of axes in graphs and the duration of reactions.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the necessity of a threshold for radical concentration or the specifics of the reactions discussed. Multiple competing views and uncertainties remain regarding the conditions affecting pollutant degradation.
Contextual Notes
Limitations include a lack of specific information about the pollutants and oxidants involved, as well as missing details in the OP's data presentation, which may affect the clarity of the discussion.