Magma828
- 28
- 0
A simple mercury barometer consists of a vertical glass tube sealed at its upper end containing a column of mercury. The space between the top of the mercury column and the upper end of the tube is a vacuum. A mercury column height of 760 mm is equivalent to atmospheric pressure 1.0x105 Pa.
A school experiment to find atmospheric pressure befotre the days of modern health legislation was as follows. A length of air was trapped in a capillary tube sealed at one end by a thread of mercury. When the tube was held horizontally, the length of the trapped air column was 82 mm and the length of the thread of mercury was 39 mm.
When the tube was held vertically with the open end upwards, the air column was squashed to 78 mm.
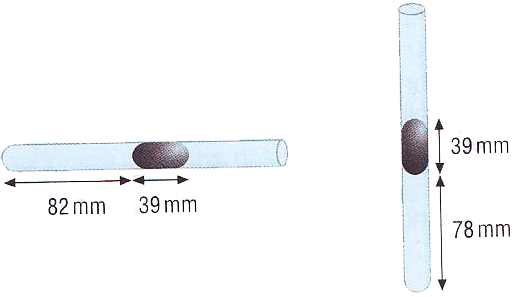
Find the value of atmospheric pressure in mm of mercury (mmHg).
We're given this statement (Boyle's Law):
The volume of a fixed mass of gas is inversely proportional to the pressure exerted on it, provided the temperature is kept constant.
I imagine this constructs an equation like this: p1V1 = p2V2
I can't really relate this to the question though, it's probably something really simple I'm missing.
A school experiment to find atmospheric pressure befotre the days of modern health legislation was as follows. A length of air was trapped in a capillary tube sealed at one end by a thread of mercury. When the tube was held horizontally, the length of the trapped air column was 82 mm and the length of the thread of mercury was 39 mm.
When the tube was held vertically with the open end upwards, the air column was squashed to 78 mm.
Find the value of atmospheric pressure in mm of mercury (mmHg).
We're given this statement (Boyle's Law):
The volume of a fixed mass of gas is inversely proportional to the pressure exerted on it, provided the temperature is kept constant.
I imagine this constructs an equation like this: p1V1 = p2V2
I can't really relate this to the question though, it's probably something really simple I'm missing.