MexChemE
- 237
- 54
I want to model the diffusion-controlled combustion of a small carbon particle. The system I want to model is similar to this one
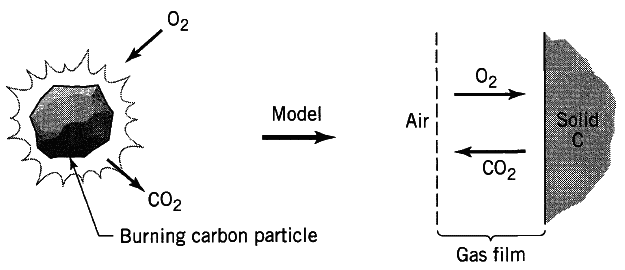
However, I'm not going to use the stagnant gas film model as shown in the figure, since I lack data for the film thickness, and I want to evaluate the problem numerically. Instead, I'm going to use spherical coordinates with boundaries r = R, the radius of the carbon particle, and r = ∞. I'm going to assign A to O2 and B to CO2 for the mathematical models. I will be using molar fractions because the global molar density of the system can be considered to be constant. The diffusion equation, concentration profile and molar flux in terms of A are given by
\frac{d}{dr} \left( r^2 \frac{dx_A}{dr} \right) = 0
x_A = x_{A \infty} \left( 1 - \frac{R}{r} \right)
N_{Ar} = - \frac{c \ D_{AB} \ x_{A \infty} R}{r^2}
Where xA∞ is the molar fraction of oxygen at r = ∞. The negative sign in the molar flux expression is there because oxygen is diffusing towards the carbon particle. My problem lies within giving a numerical value to xA∞. The statement says the particle burns in presence of air, so first I let xA∞ = 0.21, but that creates a problem. For this scenario, N_{Ar} = - N_{Br}, and if I make the analysis in terms of B, I obtain the following expressions
x_B = x_{BR} \frac{R}{r}
N_{Br} = \frac{c \ D_{AB} \ x_{BR} R}{r^2}
The problem is that xBR, the molar fraction of CO2 in the surface of the carbon particle, is always 1. Then I'm forced to assign xA∞ = 1, in order for N_{Ar} = - N_{Br} to be satisfied. The problem is that the particle is burning in air, not oxygen, so what about the presence of nitrogen? Do we just consider species A and B and neglect any other species present in the system? Do we ignore nitrogen because it is not participating in the mass transfer process? The process is diffusion-controlled, so I ignored the kinetics of the combustion and setted x_A |_{r=R} = 0 and x_{BR} = x_B |_{r=R} = 1.
My ultimate goal is to calculate the time it takes for the carbon particle to consume completely, using a quasi-steady state analysis. I have enough data to calculate this, but I want to sort this boundary condition issue out first.
The data I have are:
Carbon particle diameter: 0.05 in
Carbon particle density: 85 lb ft-3
Pressure: 1 atm
Temperature: 2500 °F
Diffusivity: 8 ft2 hr-1
Thanks in advance for any input!
However, I'm not going to use the stagnant gas film model as shown in the figure, since I lack data for the film thickness, and I want to evaluate the problem numerically. Instead, I'm going to use spherical coordinates with boundaries r = R, the radius of the carbon particle, and r = ∞. I'm going to assign A to O2 and B to CO2 for the mathematical models. I will be using molar fractions because the global molar density of the system can be considered to be constant. The diffusion equation, concentration profile and molar flux in terms of A are given by
\frac{d}{dr} \left( r^2 \frac{dx_A}{dr} \right) = 0
x_A = x_{A \infty} \left( 1 - \frac{R}{r} \right)
N_{Ar} = - \frac{c \ D_{AB} \ x_{A \infty} R}{r^2}
Where xA∞ is the molar fraction of oxygen at r = ∞. The negative sign in the molar flux expression is there because oxygen is diffusing towards the carbon particle. My problem lies within giving a numerical value to xA∞. The statement says the particle burns in presence of air, so first I let xA∞ = 0.21, but that creates a problem. For this scenario, N_{Ar} = - N_{Br}, and if I make the analysis in terms of B, I obtain the following expressions
x_B = x_{BR} \frac{R}{r}
N_{Br} = \frac{c \ D_{AB} \ x_{BR} R}{r^2}
The problem is that xBR, the molar fraction of CO2 in the surface of the carbon particle, is always 1. Then I'm forced to assign xA∞ = 1, in order for N_{Ar} = - N_{Br} to be satisfied. The problem is that the particle is burning in air, not oxygen, so what about the presence of nitrogen? Do we just consider species A and B and neglect any other species present in the system? Do we ignore nitrogen because it is not participating in the mass transfer process? The process is diffusion-controlled, so I ignored the kinetics of the combustion and setted x_A |_{r=R} = 0 and x_{BR} = x_B |_{r=R} = 1.
My ultimate goal is to calculate the time it takes for the carbon particle to consume completely, using a quasi-steady state analysis. I have enough data to calculate this, but I want to sort this boundary condition issue out first.
The data I have are:
Carbon particle diameter: 0.05 in
Carbon particle density: 85 lb ft-3
Pressure: 1 atm
Temperature: 2500 °F
Diffusivity: 8 ft2 hr-1
Thanks in advance for any input!