gpsimms
- 30
- 1
- TL;DR
- Mostly in the title: I am creating HCl in a tube reactor and crashing it out into a water trap. I am measuring pH and conductivity in the trap, and I want to say how many moles of HCl I have created.
Hi y'all,
I think this is my first time in the chemistry section of this forum, so thank you in advance for your patience with what is (I'm pretty sure) an elementary question. I've googled around for this a bit, but my last acid chemistry study was in 2002 or so, so the reading I am doing is not helping me.
I have an experiment which is creating HCl. I want to measure how much HCl my reactor is making, so the products are bubbled through a chilled water bath. I have real-time measurements of both pH and conductivity.
Once I get to low pH, the pH measurement is not sensitive enough to detect changes, due to log scale, so I also need to calculate HCl concentration from conductivity.
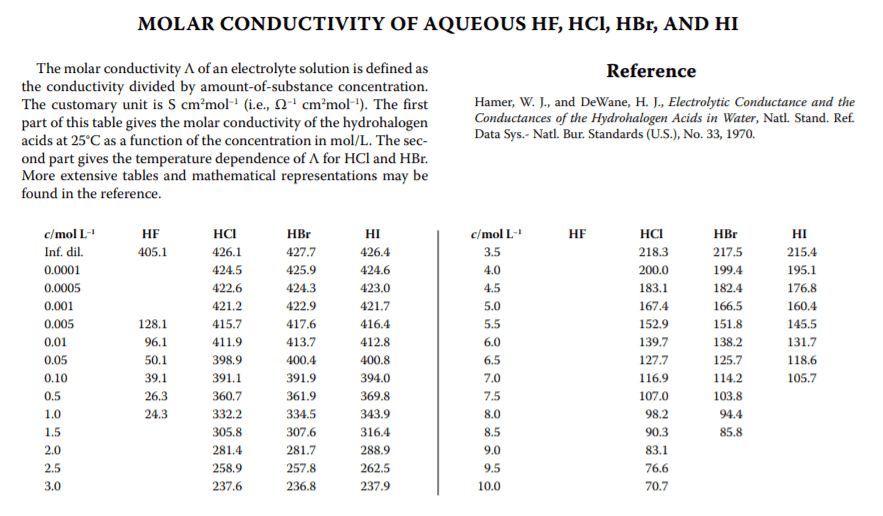
Conductivity is measured on my Hach instrument in mS/cm. Googling around led me to this table (below) for molar conductivity, but I'm a bit confused about the units. Below, it says molar conductivity is S*cm^2/mol. So does that mean that I need to take my measurement and multiply by volume of solution? And then divide by the number of moles HCl? But how do I do that, when that is my unknown?
Another question: I am starting with 2 L of H2O, and finishing with a pH around 1.6. Am I adding enough HCl that I need to consider a volume change to the water when determining concentration?
Thanks for your help!
I think this is my first time in the chemistry section of this forum, so thank you in advance for your patience with what is (I'm pretty sure) an elementary question. I've googled around for this a bit, but my last acid chemistry study was in 2002 or so, so the reading I am doing is not helping me.
I have an experiment which is creating HCl. I want to measure how much HCl my reactor is making, so the products are bubbled through a chilled water bath. I have real-time measurements of both pH and conductivity.
Once I get to low pH, the pH measurement is not sensitive enough to detect changes, due to log scale, so I also need to calculate HCl concentration from conductivity.
Conductivity is measured on my Hach instrument in mS/cm. Googling around led me to this table (below) for molar conductivity, but I'm a bit confused about the units. Below, it says molar conductivity is S*cm^2/mol. So does that mean that I need to take my measurement and multiply by volume of solution? And then divide by the number of moles HCl? But how do I do that, when that is my unknown?
Another question: I am starting with 2 L of H2O, and finishing with a pH around 1.6. Am I adding enough HCl that I need to consider a volume change to the water when determining concentration?
Thanks for your help!