Homework Help Overview
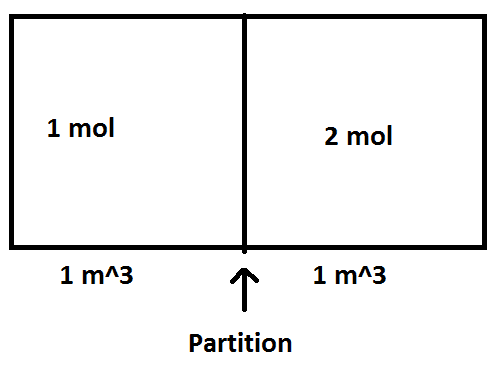
The discussion revolves around a problem involving a diatomic ideal gas (N2) in a partitioned system. Participants are tasked with calculating the change in entropy (ΔS) and the final pressure (P_final) of the gas. The initial conditions include a volume of 1 m³ for each block, a molar mass of 28 g/mol, and an initial temperature of 300 K.
Discussion Character
- Exploratory, Assumption checking, Conceptual clarification
Approaches and Questions Raised
- Participants discuss the application of the ideal gas law to find initial pressures and consider the implications of the first law of thermodynamics on internal energy and temperature. There are inquiries about how to connect internal energy changes to final temperature and the work done by the gases.
Discussion Status
The conversation is ongoing, with participants expressing confusion about the relationships between pressure, volume, and work done in the context of a rigid container. Some have suggested using specific formulas but are uncertain about the values to apply. There is a recognition that the work done may be zero due to constant volume, but further clarification is sought regarding the heat transfer and internal energy changes.
Contextual Notes
Participants are considering the constraints of the system, including whether the partition is movable or insulated, which affects heat transfer and work calculations. There is also mention of the need for specific values (like K) to proceed with calculations.