rachelmaddiee
- 67
- 5
- Homework Statement
- I have a question
- Relevant Equations
- N/A
Here is my work** Can someone please tell me if this is correct?
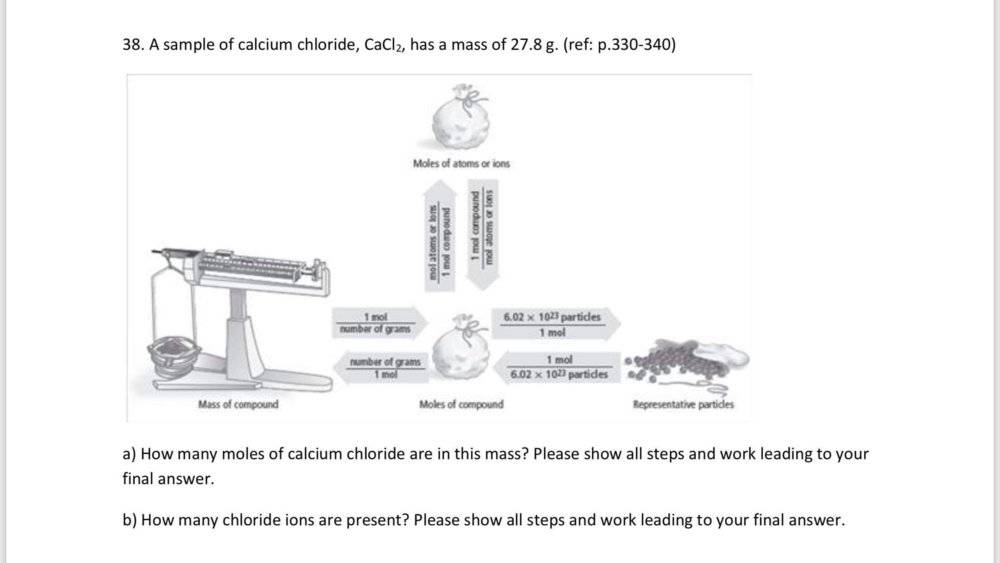
mass = 27.8 g CaCI2
Number of CaCI2 in the compound = (unknown)
Number of CI- ions in the compound = (unknown)
Number of moles = mass/molar mass
To find the number of moles of CaCI2 first you find the molar mass of the compound.
1 mole Ca x 40.08 g Ca/1 mole Ca = 40.08 g
1 mole CI x 30.45 g CI/1 mole CI = 30.45 g
1 mole CI x 30.45 g CI/1 mole CI = 30.45 g
molar mass = (40.08 g + 35.45 g + 35.45 g) = 110.98 g/mol CaCI2
27.8 g CaCI2 x 1 mol CaCI2/110.98 g CaCI2 = 0.250 mol CaCI2
Now from equation No of Chloride ions are : 6.02 x 10^23 x 2 CI- ions =
1.204 x 10^23 CI- ions
mass = 27.8 g CaCI2
Number of CaCI2 in the compound = (unknown)
Number of CI- ions in the compound = (unknown)
Number of moles = mass/molar mass
To find the number of moles of CaCI2 first you find the molar mass of the compound.
1 mole Ca x 40.08 g Ca/1 mole Ca = 40.08 g
1 mole CI x 30.45 g CI/1 mole CI = 30.45 g
1 mole CI x 30.45 g CI/1 mole CI = 30.45 g
molar mass = (40.08 g + 35.45 g + 35.45 g) = 110.98 g/mol CaCI2
27.8 g CaCI2 x 1 mol CaCI2/110.98 g CaCI2 = 0.250 mol CaCI2
Now from equation No of Chloride ions are : 6.02 x 10^23 x 2 CI- ions =
1.204 x 10^23 CI- ions
