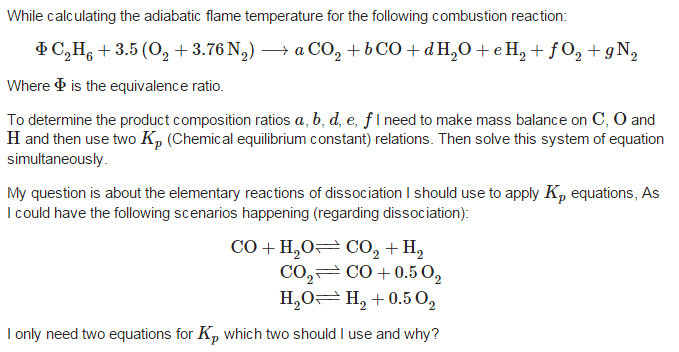SUMMARY
The discussion focuses on calculating the equilibrium constant (Kp) for simultaneous dissociation reactions in chemical equilibrium. Participants emphasize that knowing any two equilibrium constants allows for the determination of a third Kp due to the interdependence of the reactions. This principle is crucial for understanding the relationships between multiple dissociation reactions in a system.
PREREQUISITES
- Understanding of chemical equilibrium concepts
- Familiarity with the equilibrium constant (Kp) and its significance
- Knowledge of simultaneous dissociation reactions
- Basic algebra for manipulating equilibrium expressions
NEXT STEPS
- Study the derivation of the equilibrium constant expressions for simultaneous reactions
- Explore examples of calculating Kp from known equilibrium constants
- Investigate the impact of temperature on equilibrium constants
- Learn about Le Chatelier's principle and its application in equilibrium shifts
USEFUL FOR
Chemistry students, educators, and researchers interested in chemical equilibrium and reaction dynamics.